According to Cancer Research UK, there were 375,400 new cases of cancer during 2016–2018 (Cancer Research UK, 2023). Patients with cancer often suffer from acute or chronic wounds caused by either the disease itself or as a result of the cancer treatment. These wounds present many challenges for the patient, their family and the multidisciplinary team treating them.
Cancer can cause wounds in the form of multiple skin lesions or malignant fungating wounds (Naylor et al, 2001). Malignant fungating wounds are often non-healing wounds, which are caused by the aggressive proliferation of malignant cells and tumours that infiltrate the epidermis, blood vessels and underlying structures in advanced cancer patients (Grocott et al, 2013). Malignant fungating wounds can result from primary, secondary or recurring malignant disease (Alexander, 2009) and often become infected, although data on this is limited (Vardhan et al, 2019).
The wounds can be odorous, produce large amounts of exudate, bleed easily, cause psychosocial distress and are a constant reminder to the patient and family of progressive cancer (Schultz et al, 2002). The symptoms attributed explicitly to malignant fungating wounds are unique to this population (Tilley et al, 2016). Malignant fungating wounds are predominantly developed during the last months of life and indicate the impending end of life (Alexander, 2010).
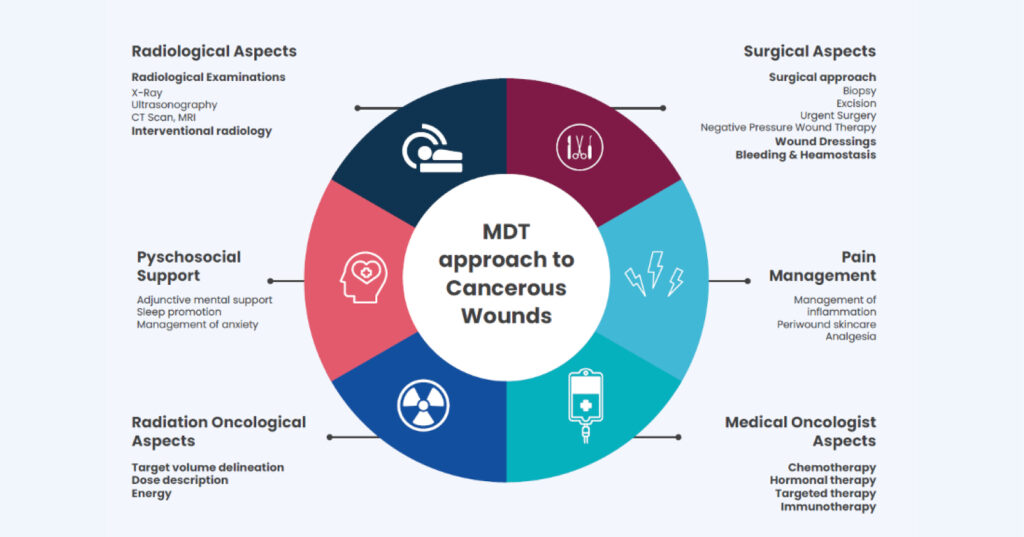
An interdisciplinary approach with continuous consultation between various specialists can support patient management (Furka et al, 2022). Patients experiencing an oncology or malignant fungating wound require a comprehensive treatment solution to alleviate severe symptoms (Figure 1).
The prevalence and challenges of malignant fungating wounds
Non-healing fungating wounds without effective therapy are a severe socio-economic burden for all involved, including patients, caregivers, and health services (Furka et al, 2022). Cancer patients with wounds may experience difficulties with symptom management, disturbances of body image, decreased feelings of self-worth and alterations in their quality of life (QoL). Such wounds often affect every aspect of an individual’s life, including work, socialisation, and relationships. This can be due to prolonged healing times, the repeated need for medical attention in the form of dressing changes, pain, infection and odour (Olsson et al, 2019).
Chronic wounds affect up to 2.21 per 1000 individuals and significantly impact rates of morbidity (Martinengo et al, 2019; Sen, 2019). It is much more challenging to assess the prevalence of malignant fungating wounds, a subset of chronic wounds, as there is no incidence monitoring registry for this type of wound.
A survey of 269 nurses in Switzerland, which was conducted over a six month period, found the prevalence of malignant wounds in patients with metastasised cancer was 6.6% (Probst et al, 2009). However, this was a nurses’ survey, not a prospective prevalence study, and the overall sample number was small, all of which means it may not represent the world population. Furthermore, the prevalence of malignant wounds is thought to be underreported due to feelings of shame, fear and embarrassment (Alexander, 2009).
A systematic review of literature published between 1995 and 2020 (Tilley et al, 2021) found that malignant fungating wounds can develop from any type of malignancy. The most prevalent are associated with breast cancer (66%), followed by head and neck tumours (24%). The groin, genitals, and back combined account for 3%, and all other sites account for 7%. According to Tilley et al (2016), malignant wounds are a global health problem and their incidence is expected to rise as the ageing population grows due to improvements in treatment.
Cancer wounds have a dynamic bacterial flora when compared with chronic wounds (Fromantin et al, 2013). Symptoms of malignant fungating wounds are already a result of an imbalance created by bacterial species, type, and total bacterial load (Vardhan et al, 2019). There is greater difficulty in diagnosing infection in malignant wounds since they often emit malodours, exudates, and necrosis, and their clinical signs are not indicative of bacterial imbalances (Fromantin et al, 2023).
A Cochrane review in 2014 found relatively little evidence for using topical agents and dressings to improve the QoL in people with malignant fungating wounds (Adderley and Holt, 2014).
In our Trust, there was an unexplainable increase of 94.4% of referrals for malignant fungating wounds in the year following the Covid-19 pandemic. This may be due to chance, or because there was a late cancer diagnosis or delayed referral during the Covid-19 pandemic. A potential gap in the education around the management of oncology wounds may also be a potential causative factor. As a tissue viability team, we wanted to raise awareness of some of the clinical issues surrounding the management of oncology wounds, as well as practical wound management recommendations for working as part of a multidisciplinary team (MDT), which could serve as a guide for healthcare professionals in future management of this wound type.
The challenge of dressing selection
According to the radiotherapy skin guidelines of the Trust, silver dressings (antimicrobial) are not appropriate to use while patients are undergoing radiotherapy. Even though there is limited evidence for the use of antimicrobials in malignant fungating wound management (Finlayson et al, 2017), antimicrobial dressings are widely used in clinical practice and are recognised to have a role in the reduction of odour (Gethin et al, 2023).
Additionally, we explored the options for dressings that do not require removal for radiotherapy treatment. In accordance with skin care guidelines, if the patient uses silver dressings for wound management, this must be removed before radiotherapy to prevent the beam from scattering. If the radiotherapy dose was calculated with the non-silver dressings on, there was no need to remove the dressings while treating the patient with radiotherapy. This indeed helps in unnecessary dressing changes.
A new chitosan bioactive microfibre gelling (BMG) dressing, MaxioCel, was recommended to our Trust for evaluatation and to determine if it can overcome the challenges staff face with dressing changes during radiotherapy. We wanted to ensure that patients received the full benefit of a dressing’s properties by not having to take them off every day, which is not necessary in most cases. The dressing’s properties include haemostatic, antimicrobial and antibiofilm actions, as well as odour and exudate management, which in turn support a reduction in inflammation and wound pain, and promotes wound healing (Table 1).
Chitosan and bioactive microfibre gelling (BMG) technology
MaxioCel is a chitosan wound dressing with BMG technology. The gelling mechanism of BMG fibres enable the dressing to maintain a cohesive structure with increased fluid absorption capacity. The dressing, with vertical wicking, is intended for the management of moderate-to-heavy wound exudate with fluid-locking ability that prevents saturation, and consequently periwound skin maceration.
Due to the absorption of bradykinin and proton ions that are released within an inflammatory site, chitosan has also been found to have analgesic properties (Mo et al, 2015). Additionally, the reduction in exudate due to the dressing’s absorption capability also improves periwound skin, which helps reduce pain levels and increases comfort levels for patients.
Grade A Chitosan is proven to have an antimicrobial effect against a range of common and uncommon wound pathogens (Dai et al, 2011). MaxioCel works in multiple ways against bacteria, including breaking down the cell wall and killing bacteria within the BMG fibres. MaxioCel’s BMG fibres are positively electrostatically charged, naturally attracting negatively charged pathogens and trapping, disrupting, and killing them within the dressing fibers.
Method
A patient evaluation was undertaken to assess the impact of the chitosan BMG dressing over a four-week period at The Christie hospital, Manchester. All patients consented to take part in the study, signing the Trust’s informed consent forms, as well as company consent forms. Consent was also given for the use of data and use of imagery for publication and teaching purposes. This was supported by the hospital’s medical illustrations department. Patient recruitment initially took place across the hospital trust, and the evaluation continued across the wider community upon discharge.
Results
Wound types included: fungating tumours to the neck (2) breast (2) sacral tumour (1) submandibular (1) groin (1) and three patients with lymphomas (3). Table 2 details the baseline demographics of the patients recruited, including wound types, location, duration, presenting factors and previous dressings used, to provide an overview of the clinical challenges faced.
By conclusion of the four-week evaluation period, the patient’s wound status was assessed as: healing (7), almost healed (2) or non-healing (1). The average wound area reduced from 51cm2 on initial assessment, to 44cm2 at final assessment.
The wound tissue type improved throughout the evaluation period, with increased re-epithelialising and granulatation tissue from 34.5% at presentation to 68.5% at final assessment. There was also a decrease in slough and necrotic tissue from 65.5% at presentation to 25.5% by final assessment (Figure 2).
By the conclusion of the evaluation period, an improvement in periwound skin condition was found with periwound maceration and excoriation reducing from 70% to 0% in the four-week period, and ‘healthy’ periwound skin increasing from 10% to 70% by the end of the evaluation (Figure 3).
Also, all patients in this study experienced a reduction in pain within the first few dressing changes, reducing in an average pain level of six on the visual analogue scale (VAS) at initial presentation, to two at final assessment (Figure 4). In the case of a 49-year-old female with T-cell lymphoma (Case study 2), the wound management objectives included the management of the patient’s pain levels while promoting healing. The patient initially reported pain level as eight on VAS, causing significant distress and impacting QoL. By the end of the four-week evaluation, the patient’s pain level had reduced to two.
Case study 1
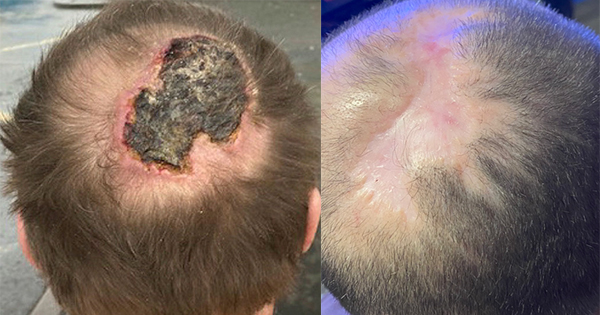
A 56-year-old female with a malignant fungating wound to the left side of her neck that had been present for 12 months. The patient was undergoing radiotherapy. Wound management objectives were to encourage granulation, reduce exudate and the associated odour, and to reduce excoriation during patient’s end-of-life care.
Full details can be found in Case study 1. In summary after the four-week treatment, the periwound skin was healthy. Community nurses were able to perform dressing changes on alternate days, which allowed the patient comfort during her end-of-life care. The patient found the dressing to be extremely comfortable and was very pleased with how it managed both exudate and in particular odour, and supported some reduction in wound pain.
Case study 2
A 49-year-old female with T-cell lymphoma on the left arm (5cm x 4.5cm) and satellite wound (3.5cm x 3.5cm). The patient expressed that the wound was impacting her QoL, as the offensive malodour of the wound was causing her embarrassment at work, where there was a shared office environment. Objectives were to manage the patient’s pain levels, promote healing, protect granulation tissue, manage exudate and support autolytic debridement.
Full details can be found in Case study 2. In summary, within 4 days of begininng treatment using the chitosan BMG dressing, pain reduced, the necrotic area was debrided, slough softened, odour reduced, and dressing change reduced from 2–3 times per day to daily, allowing supported self-care. The periwound skin had improved. The patient requested to continue with the dressing beyond the evaluation. She was also receiving immunotherapy treatment.
Discussion
In patients with malignant fungating wounds, an aggressive approach of debridement, cleansing and application of topical antimicrobial may not be possible due to patients’ levels of pain and discomfort. The chitosan BMG dressing facilitated gentle debridement and minimisation of odour as a result of removing the bacterial load from the wound. Evidence of the dressing facilitating autolytic debridement can be seen in Case study 1, where the dressing supported the end-of-life care. Working in partnership with both radiotherapy and chemotherapy colleagues, and their associated treatment regime, also contributed to outstanding results in a relatively short period of time.
Malignant wounds have a myriad of unpleasant symptoms including odour, pain, bleeding and excessive exudate. The individual with a malignant wound can suffer physical and social distress due to the unacceptable and offensive presence of the wound. Normality is turned on its head and they find themselves in a painful and undesirable situation (Young, 2017). By being able to manage the patient’s wound pain, promote wound progression across the healing continuum, in the majority of cases, and together with end-of-life care, this evaluation demonstrated an enhanced experience for all involved and increased all the patient’s QoL
This work has been presented at a national virtual oncology conference to support other clinicians managing oncology wounds alongside two virtual symposia. It is hoped that the introduction of a pathway (Figure 5) will support other clinicians in the management of their patients’ oncology wounds and facilitate further discussion, education and training events around this topic in the future.
Conclusion
The case study series has been a thought-provoking experience for both patients and staff across all departments and specialities. The impact that the introduction of BMG Fibre technology has had alongside chemotherapy and radiotherapy, in such a short period of time, has been remarkable.