Electrical stimulation (ES) can be used to stimulate healing in hard-to-heal wounds in conjunction with standard wound management, such as compression therapy. ES therapy has been used for more than 30 years to facilitate wound healing but, historically, its use in clinical practice has been perceived as problematic and complex and key ES research findings have not been clearly interpreted. This made easy looks at Accel-Heal® (Medicareplus International Ltd), a modern device that delivers a condition-specific microcurrent treatment programme for non-healing and poorly healing wounds in order to stimulate healing.
Why some wounds don’t heal
A hard-to-heal wound is defined as one that fails to heal with standard therapy in a timely fashion (Troxler et al, 2006). Normal wound healing starts with haemostasis, progresses through a destructive inflammatory phase and then a restorative phase. It finishes with remodelling of the wound. This process can be interrupted at any stage due to a number of intrinsic and extrinsic factors (Vowden, 2011). For patients in which healing is prolonged, clinicians face the dual challenge of managing the wound environment and the patient, whose wellbeing may be significantly compromised (EWMA, 2008).
Treating hard-to-heal and long duration wounds is costly, and impacts on staff time and product use. To ensure good symptom management (eg pain or exudate) and minimise healthcare costs, it is important that hard-to-heal wounds are identified early. A careful wound and patient assessment can identify intrinsic and extrinsic factors that can delay healing, such as ischaemia, infection and underlying comorbidities. Recognising non-healing requires careful reassessment over several weeks while delivering a treatment plan to move the wound towards healing (Troxler et al, 2006). If the wound fails to reduce in size or there is no improvement within the expected timeframe, it is essential to reassess the patient and alter the treatment plan.
The role of advanced wound therapies
Wound bed preparation is recognised as having an important role in promoting healing and preventing the breakdown of the extracellular matrix (ECM) in wounds that are failing to heal (Schulz, 2009). The ECM is the major structural component of the dermis and primarily contains collagen, which provides support for cells, growth factors and receptors essential for wound healing. Introducing advanced wound therapies can be considered in patients who are not responding to standard therapy. This can result in improved symptom control, healing rates and reductions in long-term healthcare costs, despite the initial outlay for treatment, which might seem significant (Vowden, 2011).
What is Electrical Stimulation?
The interactions and effects electricity has on biological and physiological processes are many, varied and complex. The cell membrane has been identified as a key point of interaction with an externally applied current (Lee et al, 1993). As far back as 1850 it was recognised that the body generates natural electrical fields (Kloth, 2005). These create positive and negative charged ions that transfer across wound tissues and generate an electrical current (Vanable 1989). This endogenous ‘skin battery’ is believed to produce an electrical current in response to the resistance of the skin (Foulds and Barker 1983). When injury occurs (eg a break in the skin) there is a break in the continuity of the current resulting in the electrical potential being discharged as an electric field (Barker et al, 1982; Vanable, 1989; Jaffe and Vanable 1984).
It is believed that the discharge of current (known as the ‘current of injury’) helps to orchestrate tissue repair by attracting different cell types into and across the wound, stimulating cell proliferation and collagen synthesis (Tadej et al, 2010) and activating specific gene expression important in tissue repair (Zhao et al, 2004). This current of injury extends up to a radius of 2-3mm around the wound (Tadej et al, 2010). As the wound closes, the current of injury progressively reduces. In wounds in which healing is delayed, the current of injury is disrupted. Dry wounds, for example, have increased electrical resistance, resulting in the current being ‘switched-off’ (Cheng et al, 1995). Non-healing wounds also show a lack of electrical activity (Kloth and McCulloch, 1996).
ES therapy involves the transfer of an electrical current to the skin surface adjacent to the wound, creating a flow of ions through the wound tissue (Chapman-Jones et al, 2010).

Evidence for ES and wound healing
Stimulation of wounds with an external electric current has been investigated as a potential method for promoting or stimulating the wound healing process. It has been shown that externally-applied electrical currents can induce changes capable of increasing healing potential and the rate of wound repair. The exact mechanisms of some of these effects are still being investigated, but the most notable outcomes include:
- Faster wound closure (van Rijswijk, 1993; Arnold et al, 1994; van Rijswijk and Polansky, 1994; Robson et al, 2000)
- Increased migration of neutrophils, macrophages and fibroblasts (Assimacopoulos, 1968a, 1968b; Ottani et al, 1988)
- Increased angiogenesis and capillary density (Zhao et al, 2004)
- Increased blood flow and oxygenation (Gagnier et al, 1988; Peters et al, 1998)
- Increased fibroblast proliferation and activity (Gagnier et al, 1988; Peters et al, 1998; Sugimoto et al, 2012)
- Decreased oedema (Mohr et al, 1987; Reed, 1988; Chapman-Jones et al, 2010)
- Increased collagen synthesis and tensile strength (Assimacopoulos, 1968; Kloth, 2005)
- Disruption of biofilms and reduced bacterial proliferation (Kincaid and Lavoie, 1989; Moore, 2007)
Use of ES in clinical practice
Historically, the use of ES in clinical wound care practice has been problematic. Many units were large and needed to be attached to the patient several times a day, often requiring a mains electrical supply. This would pose difficulties for patients who were cared for in the community, who were seen for 10-15 minutes twice a week. In addition, the need for health professionals to set therapeutic delivery parameters adversely affected the adaptability and cost of ES therapy. The introduction of the Accel-Heal® system has been designed to overcome these difficulties and can be fully integrated into existing care packages.
What is Accel-Heal®?
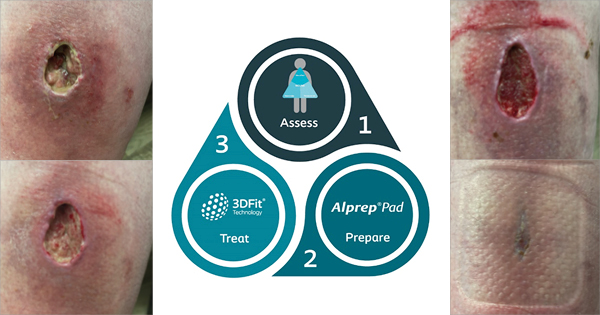
Accel-Heal® is a registered class IIa medical device which delivers low-level ES therapy for use in the management of chronic, non-healing wounds. Each unit comprises a single-use, disposable current generator that is applied using a pair of disposable electrodes. The system delivers a pre-set series of low-intensity electrical pulses which deliver a microcurrent to the wound.
When to use Accel-Heal®
Accel-Heal® is indicated for patients with a hard-to-heal venous leg ulcer of over six months’ duration. It can be applied at any stage of healing and is an adjunct therapy to be used alongside existing management strategies. It can be used under compression bandaging.
Contraindications
Accel-Heal® should not be used for patients with active cancers. Some patients might have an allergic reaction to the pads, although this is rare. The electrode pads should not be placed directly over broken capillaries or varicose veins.
Clinical application of Accel-Heal®
How to apply Accel-Heal®
The electrode pads are placed close to the wound border on either side of the wound (Fig 1). The thin electrical cables can be placed under dressings or bandages and are connected to the current generator, which is placed into the bandage or patient’s clothing (Tadej et al, 2010). The device is activated by pressing a button and generates a pulsed microcurrent in a preset sequence. This current is below the threshold needed to stimulate muscle or nerve activity and so goes undetected by the wearer. The electrode pads conduct the naturally changing resistance of the skin to the unit and it automatically adjusts its electrical output to ensure continuous delivery of the required therapeutic current.
How long to use Accel-Heal®
A standard Accel-Heal treatment programme runs for 12 days and uses six device units (varying numbers of units have been used in trials and evaluations but a 12-day treatment programme is recommended). Once activated, each unit delivers therapy continuously for 48 hours of treatment. When a unit reaches the end of its preset therapy sequence it can be changed either by the patient, his/her carer or by a health professional at the next dressing change.
On average, wounds smaller than 15cm2 require six Accel-Heal® units to achieve effective treatment outcomes. For wounds larger than 15cm2 it has been suggested that Accel-Heal® be used until the wound has reduced in size by 50% (Chapman et al, 2010).
When should I see an improvement in the wound?
Although progress may be seen immediately, Accel-Heal® is not expected to heal the wound within the 12-day treatment programme. However, the therapy ‘kick starts’ the healing process, while the management regimen is continued or modified. A predictive model has shown that wounds less than 15cm2 might be expected to heal within an 8-week period (Chapman et al, 2010).

Evidence For Accel-Heal®
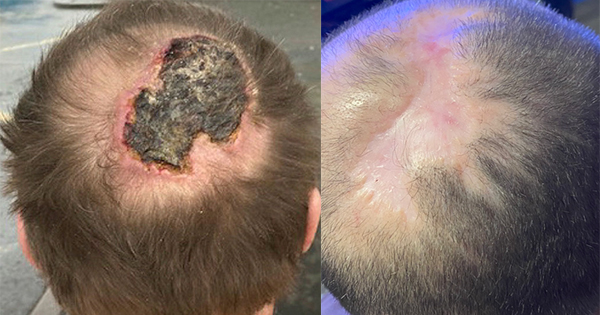
Young et al (2011) undertook a non-blinded, clinical evaluation using Accel-Heal® of 30 patients with full thickness, venous leg ulcers that had been non-healing for more than 6 months. The wounds were formally assessed and confirmed as non-healing for two months prior to the evaluation of Accel-Heal®.
All patients were treated for 10 days and patients’ wounds were monitored over a 3-month period. During this time periwound oedema had decreased by approximately 60% of the original level. This was maintained at 90 days (Young et al, 2011). In addition mean pain levels had reduced to 1.6 from 5.3 (using an 11-point score of 0=no pain and 10=worst pain) over the treatment period (Tadej et al, 2010). Exudate levels also reduced (with 51.7% lower fluid loss) at the end of the treatment period.
Chapman-Jones et al (2010) reported that 95% of chronic, non-healing venous leg ulcers studied had improved at 90 days, with 38% of going on to achieve full closure within 19 weeks. Further studies are needed to confirm the benefits of using Accel-Heal®.
Clinical benefits of Accel-Heal®
Improving rates of healing can have significant benefits for the patient: with a reduction in pain and exudate levels and improved quality of life (EWMA, 2008). Preliminary results may suggest that the device can have a positive impact on quality of life and can help to manage symptoms effectively, such as pain, exudate and oedema.
Patients can wash with the device in place. They may shower but the unit must be detached from the electrical cables. Patients’ reliance on carers and nurse visits is reduced, allowing them to feel more in control and visualise a future without a wound (International Consensus, 2012).

Cost benefits of Accel-Heal®
Taylor et al (2011) undertook a formal cost-analysis of the use of Accel-Heal® on non-healing venous leg ulcers. They assumed that three devices were used per patient at a cost of £40 each. Using a Markov model, they demonstrated that Accel-Heal® was a cost-effective treatment that could produce savings. Despite the £240 upfront investment for six Accel-Heal® devices, the five-month healthcare costs of using Accel-Heal® plus dressings and compression bandaging was given as £748.94, versus £879.90 for dressings plus compression bandaging alone (Taylor et al, 2011). Use of the product as an adjunct to appropriate topical management was estimated to achieve a 27% reduction in nurse visits, a 56% reduction in bandage usage and a 27% reduction in dressings leading to a 15% reduction (£6.2 million) in total costs to the NHS.
Summary
When the body’s endogenous bioelectric system fails and cannot contribute naturally to the repair processes, introducing therapeutic levels of electrical current into the wound tissue from an external source may help to stimulate healing. This treatment has been found to be useful in hard-to-heal wounds and chronic venous leg ulcers in particular. Accel-Heal® provides a simple, safe and potentially cost effective method of ES treatment, offering benefits to patients and healthcare services alike.
References
- Arnold TE, Stanley JC, Fellows EP, Moncada GA, Allen R, et al (1994) Prospective, multicenter study of managing lower extremity venous ulcers. Ann Vasc Surg 8: 356-362
- Assimacopoulos, D (1968a) Low intensity negative electric current in the treatment of ulcers of the leg due to chronic venous insufficiency. Preliminary report of three cases. Am J Surg 115(5): 683–7
- Assimacopoulos D (1968b) Wound healing promotion by the use of negative electric current. Am Surg 34(6): 423-31
- Barker A, Jaffee L, Vanable J Jr (1982) The glabrous epidermis of cavies contains a powerful battery. Am J Physiol 242: R258–66
- Chapman-Jones D, Young S, Tadej M (2010) Assessment of wound healing following electrical stimulation with Accel-Heal. Wounds UK 6(3): 67-71
- Cheng K, Tarjan P, Oliveira, Gandia M et al (1995) An occlusive dressing can sustain natural electric potential of wounds. J Invest Dermatol 104(4): 662-5
- European Wound Management Association (EWMA) (2008) Position document: hard-to-heal wounds: a holistic approach. MEP Ltd, London
- Foulds IS, Barker AT (1983) Human skin battery potentials and their possible role in wound healing. Br J Dermatol 109: 515-22
- Gagnier KA, Manix N, Baker L, et al (1988) The effects of electrical stimulation on cutaneous oxygen supply in paraplegics. Phys Ther 68(5): 835-9
- International Consensus (2012) Optimising wellbeing in people living with a wound. An expert working group review. London: Wounds International. Available from: www.woundsinternational.com
- Jaffe L, Vanable JW Jr (1984) Electrical fields and wound healing. Clin Dermatol 2(3):34–44
- Kincaid CB, Lavoie KH (1989) Inhibition of bacterial growth in vitro following stimulation with high voltage, monophasic pulsed current. Phys Therapy 69(8): 651-55
- Kloth LC (2005) Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 4(1):23-44
- Kloth LC, McCulloch JM (1996) Promotion of wound healing with electrical stimulation. Adv Wound Care 9(5): 42-5
- Lee RC, Canaday SB, Doong H (1993) A review of the biophysical basis for the clinical application of electric fields in soft-tissue repair. J Burn Care Rehabil 14(3): 319-333
- Mohr T, Akers TM, Landry RL (1987) Effect of high voltage stimulation on edema reduction in the rat hind limb. Phys Ther 67(11): 1703-8
- Moore K (2007) Electrical stimulation for treatment of chronic wounds. J Community Nurs 21(1): 1-6
- Ottani V, De Pasquale V, Govoni P, et al(1988) Effects of pulsed extremely low frequency magnetic fields on skin wounds in the rat. Bioelectromagnetics 9: 53-62
- Peters EJ, Armstrong DG, Wunderlich RP, et al (1998) The benefit of electrical stimulation to enhance perfusion in persons with diabetes mellitus. J Foot Ankle Surg 37(5):396-400, 447-8
- Reed BV (1988) Effect of high voltage pulsed electrical stimulation on microvascular permeability to prasma protein: a possible mechanism in minimizing edema. Phys Ther 68: 491-5
- Robson MC, Hill DP, Woodske ME, Steed DL (2000) Wound healing trajectories as predictors of effectiveness of therapeutic agents. Arch Surg 135(7):773-777
- Sugimoto M, Maeshige N, Honda H, et al (2012) Optimum microcurrent stimulation intensity for galvanotaxis in human fibroblasts. J Wound Care 21(1):5-6, 8,10
- Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen;17(2):153-62
- Tadej M, Young S, Hampton S (2010) Accel-Heal®: a new therapy for chronic wounds. J Comm Nursing 24(5)16-20
- Taylor RR, Sladkevicius E, Guest JF (2011) Cost effectiveness of electrical stimulation therapy in managing chronic non-healing venous leg ulcers. J Wound Care 20(10):464-72
- Troxler M, Vowden K, Vowden P (2006) Integrating adjunctive therapy into practice; the importance of recognising ‘hard to heal’ wounds. World Wide Wounds. Available from: www.worldwidewounds.com/2006/december/Troxler/Integrating-Adjunctive-Therapy-Into-Practice.html.
- Vanable JW (1989) Integumentary potentials and wound healing. In: Borgans R et al (eds) Electrical Fields in Vertebrate Repair. Alan R Liss, New York: 183
- van Rijswijk L (1993) Full-thickness leg ulcers: patient demographics and predictors of healing. Multi-center leg ulcer study group. J Fam Pract 36(6):625-632
- van Rijswijk L, Polansky M (1994) Predictors of time to healing deep pressure ulcers. Ostomy Wound Manage 40(8):40-42, 44, 46-48
- Vowden, P (2011) Hard-to-heal wounds made easy. Wounds International 2(4). Available at: http://www.woundsinternational.com/made-easys/hard-to-heal-wounds-made-easy
- Young S, Hampton S, Tadej M (2011) Study to evaluate the effect of low-intensity pulsed electrical currents on levels of oedema in chronic non-healing wounds. J Wound Care 20(8):368, 370-3
AUTHOR DETAILS
Butcher M[1], Tadej M[2], Hampton S[3]
1. Tissue viability nurse and associate lecturer, University of Plymouth
2. Tissue viability and research nurse, Eastborne Wound Healing Centre CIC
3. Tissue viability consultant, Eastborne Wound Healing Centre CIC