Wound healing is the skin repair process that occurs in response to skin/tissue injury and involves a complex interplay of several physiological systems (NLM, 2023). A typical wound heals within 4–6 week. However, the wound could become chronic if the molecular pathways involved in healing fail to progress within this timeframe (NLM, 2023). The wound prevalence is rising in the UK and represents an unmet need to find treatments that can speed the management of chronic wounds and can save time for primary care workers (Guest, 2021).
Injured or compromised skin is repaired via four distinct physiological phases: haemostasis, inflammation, proliferation and remodelling; however, wounds can become chronic/hard-to-heal if these phases do not occur in a timely manner, resulting in a wound that becomes stuck in the inflammatory stage (Snyder et al, 2020).
This may happen because of anti-inflammatory cells converging on the wound due to injury, infection and extracellular matrix fragments/proteases, keeping the wounded area inflamed (Snyder et al, 2020). Some conditions, such as diabetes, can also impair the timely progression of the healing process (Paige et al. 2019).
Macrophages as inflammation markers for chronic wounds
Macrophages, the mononuclear phagocytes employed by immune system, play a crucial role in wound healing by reducing inflammation and encouraging tissue remodelling in injured skin (Frykberg and Banks, 2015; Strizova et al, 2023). Upon reaching the wound’s microenvironment, the macrophages switch into two main phenotypes, M1 and M2 [Figure 1a; adapted from Snyder et al. 2020 and Paige et al. 2019]. The M1 cells secrete cytokines and promote phagocytosis and inflammation that the wounded area needs for eliminating bacteria and debris. In this ‘cleaner’ environment, the M1 macrophages transition to the M2 phenotype, which appear to drive the remodelling and proliferation of wounded tissue. The ratio of M1:M2 macrophages can, therefore, indicate the level of inflammation and wounds that fail to heal appear ‘stuck’ in the M1 phenotype, indicated by a high M1:M2 ratio [Figure 1b; Sahin et al, 2017]. In diabetes, the M1 phase is prolonged and progression to the M2 phase is suppressed, making people with diabetes prone to chronic wounds (Aitcheson et al, 2021).
Extracellular matrix-based therapies for chronic wounds – the urinary bladder matrix (UBM)
In chronic wounds, where inflammation and proteolysis impair the extracellular matrix function, (Snyder et al, 2020), bioengineered natural or synthetic skin substitutes from both animals and humans have emerged as a promising treatment: they provide a scaffold that changes the wound microenvironment and promotes healing (Snyder et al, 2020; Savoji et al. 2018). UBM is a skin-substituting acellular therapy [Figures 2a and b; example shown: the Integra MicroMatrix®], which in patients with diabetes, has been shown to promote healing, potentially by promoting M2 macrophages and by providing a scaffold for multiplication of new cells (Savoji et al. 2018). In a study of wounds in people with diabetes, Paige et al (2019) reported a significant decrease in the M1:M2 ratio after UBM treatment and showed that the diabetic wounds underwent a greater M1:M2 ratio decrease compared with non-diabetic wounds [Figures 2c and 2d; Paige et al. 2019]; the extent of this M1:M2 ratio reduction was also corelated with the rate of decrease in the wound area (Paige et al. 2019).
Aim of the current study – the Integra MicroMatrix® treatment for chronic, diabetic wounds
In his presentation, Jeffery explained that UBMs, in the particulate form MicroMatrix®, have traditionally been used in operating theatre environments and there is an unmet need to assess the use of MicroMatrix® as a primary treatment for chronic/hard-to-heal wounds, especially in frontline care. Therefore, in this patient case study (n=3), his team evaluated the effect of powdered or paste-form MicroMatrix® via a wound treatment regimen that can be adapted for nurse-led services across the NHS.
Methods and data collection
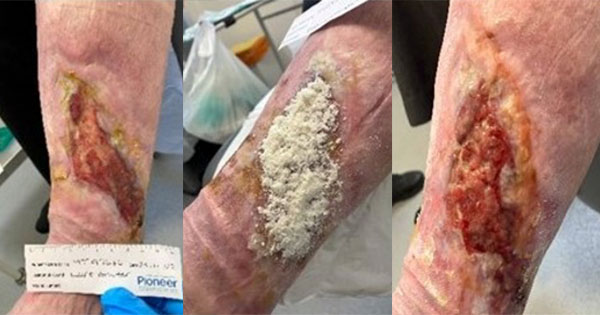
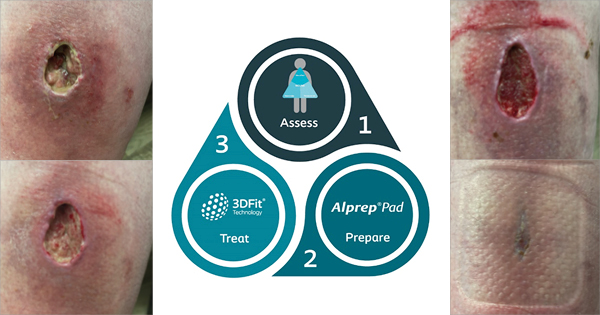
Jeffery presented outcome data from three patients with diabetes anonymised to A, B and C, respectively. The UBM used in this study was the MicroMatrix®, supplied by Integra [Figures 2a and b]. Jeffery notes that the coarse nature of the UBM powder increases surface area exposed to the wound’s microenvironment. The schematic in figure 2a shows the treatment algorithm employed to dress three chronic, stalled wounds in patients A, B and C. Before applying the MicroMatrix®, it was ensured that the wound contained no infection and was moist.
Patient outcomes
Following results summarise the results shared by Jeffery during the presentation.
Patient A: This 70-year-old patient with diabetes had a long-standing ulceration that had initially responded well to a 10-month treatment plan (compression and weekly curettage; ankle brachial pressure index 1.1) [Figure 3, top]; thereafter, the wound became static and deepened for approximately 3 months, increasing by 50% compared with the first assessment, before the UBM treatment was initiated [Figure 3, bottom]. Patient A experienced significant wound restimulation and rapid reduction in wound depth after receiving the UBM treatment for five weeks (33%, 41% and 70% reduction in wound length, width and depth, respectively, compared with the wound size at UBM treatment initiation).
Patient B: This patient with diabetes had a chronic wound (~9 years) and, despite receiving a weekly treatment comprising sharp debridement and full-compression bandages, was experiencing alternating stages of slow healing and statis. Overall, patient B had experienced no discernible physical changes in the wound dimensions for >1 year before the MicroMatrix® treatment was initiated [Figure 4; top] resulting in a moderate decrease in wound dimensions and improvement of wound bed [Figure 4; bottom]; after five weeks, the wound dimensions appeared remodelled, and granulation tissue improved (10%, 33% and 0% reduction in wound length, width and depth, respectively, compared with the wound size at MicroMatrix® treatment initiation).
Patient C: This 72-year-old patient with diabetes had a hard-to-heal lower-leg ulceration that had become static for approximately four months despite regular, standard treatment with routine debridement and full-compression in an optimised wound bed [Figure 5, top; image taken at the 3-month timepoint]. The patient then received the MicroMatrix® treatment with continued compression; debridement was halted at this stage for two weeks to optimise healing. At four weeks, granulation islands developed around the central wound bed [Figure 5; bottom]; this was followed by persistent healing recorded at 6 weeks (54%, 72% and 100% reduction in wound length, width and depth, respectively, compared with the wound size at MicroMatrix® treatment initiation).
Presentation conclusion and audience discussion
Jeffery concluded that, based on these case studies, using MicroMatrix® may be an effective treatment for chronic wounds in people with diabetes and that its ease of use may improve the cost of chronic wound management in primary care.
After the presentation, there was a lively discussion that ensued with a number of audience questions regarding the use of MicroMatrix®. A snapshot of the audience Q&A session is available in table 1.
Summary and conclusion
Wound management costed NHS an estimated £8.3 billion in 2017/2018 (Guest, 2021). This current study presents the Integra MicroMatrix® as a promising treatment for chronic, hard-to-heal wounds where inflammation prevails, and shows that MicroMatrix® can be adapted to use in a nurse-led setting at the frontline in the NHS.
Jeffery concluded the presentation with an emphasis on the emotional and financial costs of current chronic wound management methods versus an intervention with MicroMatrix®. He envisages a future where newer interventions for chronic wounds can be used by all frontline healthcare professionals because the realm of experimental treatments is currently only within the reach of surgeons and specialists. This reduces the potential impact at the frontline where nurse-led care – arguably the biggest time spend in wound management in
NHS – predominantly occurs. Therefore, nursing-led community care should be empowered so more recent interventions can be employed reduce wound management cost to the NHS.
Conflict of interest
Please note this paper is a summary of the presentation given; it is not a full publication of the clinical data and as such has not undergone peer-review. The speaker has declared no conflict of interest in conducting this study.