According to the World Health Organization (WHO), it is estimated that each year, approximately 11 million people suffer from burn wounds (Markiewicz-Gospodarek et al, 2022) and invasive infection is now the chief reason for death after burn injuries, with infected burn wounds being responsible for 51% of burn related deaths (Norbury et al, 2016). Despite advances in burn wound management, infection is a primary cause of morbidity in all age groups. One of the organisms responsible for infections in burn wounds is Pseudomonas aeruginosa, an opportunistic pathogen capable of infecting most tissues and commonly demonstrating low susceptibility to therapeutic modalities. Another organism of particular concern for burn patients is the multidrug resistant pathogen Staphylococcus aureus, which expresses an ability to excrete several protein exotoxins that can lead to additional complications. With the growing threat of multidrug resistant pathogens in burn injuries, novel therapeutic innovation remains important (Salyer et al, 2021).
Biofilm and burn wounds
Biofilms are matrix-enclosed communities of bacteria that are significantly less susceptible to host defences and to conventional therapies compared with their planktonic counterparts. The National Institutes of Health (NIH) estimate that biofilms play a role in over 80% of bacterial infections (NIH, 2022). A study published by the UK’s National Biofilms Innovation Centre (NBIC) determined the impact of biofilms within the wound care sector accounted for $281 billion annually (Cámara et al, 2022). As in chronic wounds, burn wounds with residing biofilm infections are typically more challenging to resolve than non-biofilm encased infections. The reduced clinical outcomes, and the enormous health and financial burdens of infection in burn wounds, mean that understanding the interaction of infection treatments with biofilm infection, in burn wound pathogenesis, is a major research priority (Salyer et al, 2021).
Opportunistic pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus and Acinetobacter baumannii are notorious for colonising burn wounds. The ability of these pathogens to form biofilms is a major contributor to their pathogenic success and further complicates burn wound management (Maslova et al, 2021). Wound care dressings, claiming antibiofilm therapeutic features, are often applied to burn wounds to inhibit bacterial growth and replication (Figure 1). To assess the ability of an antimicrobial agent to prevent biofilm formation or breakdown an established biofilm, it is first important to be able to grow reproducible, clinically relevant biofilms.
Some bacteria have the capacity to resist and attach to surface in the body. This is the scenario where biofilm gets involved in a chronic infection. At this level, bacterial populations can adapt to the host and survive. Several different in vitro, in vivo and ex vivo wound models have been developed to mimic biofilm infections within chronic wounds. Chronic wounds are highly complex and multiple different factors must be taken into consideration, including physiochemical and human-supplemented factors. When using alternatives to in vivo models, limitations such as the lack of a responsive immune system should always be given due consideration (Thaarup et al, 2020), however, the use of in vitro and ex vivo models can greatly improve product screening and development assessments.
Comparison of common burn wound models
It has been reported that of the commonly available burn wound models 74% are performed in vivo, 23% are in vitro and just 3% use ex vivo tissue (Thomas et al, 2021). The majority of burn wound models used for preclinical testing are in vivo, most often these are porcine studies (Figure 2) — due to the close relevance to human skin and immune system — in addition rat and mouse models are also commonly used. In vivo models can encompass burn wounds, full- or partial-thickness wounds, and diabetic ulcers, but for the purpose of this review, we’ll be focusing on burn wound models. In vivo studies have significantly enhanced the understanding of burn wound biofilm formation. However, in vivo research is not ethically popular, can be expensive and in vivo animal studies have their own unique drawbacks for modelling human infection (Uttekar et al, 2023).
In vivo models help answer pivotal questions facing the research field such as understanding the distinction between transient colonisation and infection as well as the role of biofilm formation in acute infection (Maslova et al, 2021). In order to address chronic wound healing challenges, appropriate biofilm models are necessary. To this end, several model systems (in vivo, in vitro and ex vivo) mimicking the conditions observed in a biofilm infected chronic wound have been developed.
In many cases, before in vivo studies are considered, in vitro assays are used to screen potential treatments and to establish basic antimicrobial mechanisms. A major advantage of using in vitro assays is that they can be easily replicated, providing reproducibility and allowing for statistical analysis of the results. This reproducibility helps researchers validate their findings and draw robust conclusions about the biological mechanisms they are investigating. Additionally, in vitro assays can be scaled up to screen large numbers of compounds or test multiple conditions simultaneously, facilitating high-throughput analysis and accelerating the discovery of novel mechanisms (Figure 3). Conversely, while many in vitro models are simple, affordable, and scalable, they often provide a poor representation of the wound environment, largely restricting growth of biofilms to abiotic surfaces (Alves et al, 2018).
Basic in vitro models do not fully mimic the complexities of a clinical burn wound. There is a high demand for alternative, robust and affordable methods that can provide relatable and reproducible results when testing topical treatments, both in research and in the pharmaceutical industry (Andersson et al, 2021). Ex vivo porcine skin models provide a strong representation of human skin, without the ethical and financial factors that come with animal testing (Table 1). The models outlined in this review have differing advantages and challenges that should be considered in the context of the biological question being asked to reduce and refine the use of live animals in burn wound research and to maximise the scientific outputs (Maslova et al, 2021).
Advantages of an accredited ex vivo burn wound model
These models are typically based on tissues and organs collected from living organisms, and experiments are then conducted under artificial conditions. The use of ex vivo models is increasing (Andersson et al, 2021) because they allow for more controlled experimental conditions and are covered by fewer ethical concerns when compared with in vivo studies, while also representing more clinically relevant conditions compared with in vitro methods (Andersson et al, 2021). Development of innovative burn wound care therapeutics, using ex vivo models provides robust, reproducible data to support clinical trials, while avoiding the often-higher cost and longer timelines associated with in vivo methods.
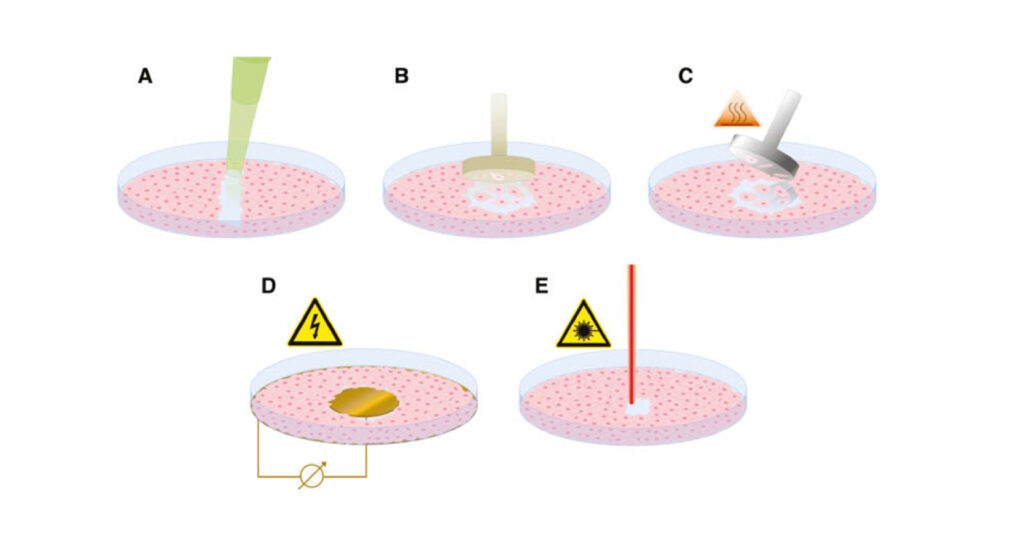
An ex vivo burn wound model has been developed, by the University of Bath, to grow reproducible biofilms on the surface of porcine skin, and further developed commercially by Perfectus Biomed Group, NAMSA, achieving ISO 17025 certification and UKAS accreditation in May 2022. The method involves the growth of repeatable Pseudomonas aeruginosa and Staphylococcus aureus biofilms on individual burn wounds on the surface of ex vivo porcine skin. This model mimics a scenario where biofilm exists within burn wounds. The methodology requires burn wounds to be formed on sterile porcine skin (of animals destined for human consumption), using a custom-created tool. Wounds were inoculated with Pseudomonas aeruginosa or Staphylococcus aureus at a known concentration and incubated at 37±2°C for 72 hours (Figure 4). The mature biofilms were treated for 24±2 hours with a range of treatment types including negative control, antibiotic controls, and positive controls. Organisms that remained after treatment were enumerated.
The importance of this model being ex vivo should not be underestimated as it allows for the development of bacterial communities more like those in a clinical burn wound. The significance of a model that can differentiate product efficacy both within and between product groups is a critical tool in helping companies to screen appropriate formulations, and helping healthcare professionals to confidently make choices around products that are likely to be effective within clinical settings.
Preclinical testing to compliment the diversity and complexity of antimicrobial wound dressings
As discussed, there are staggering statistics that link infection to mortality. Burn injury disrupts both the normal skin barrier and many of the host defence mechanisms that prevent infection. This makes burn wounds potentially susceptible to colonisation and infection by the multitude of environmental microorganisms with which the human body normally coexists. Therefore, management of severe burns requires the suppression of microbial growth, particularly when eschar and damaged tissue are present (Parikh et al, 2005). Widespread application of effective antimicrobial agents reduces the microbial load on the burn wound surface and reduces the risk of infection (Dai et al, 2010). There is a wide range of advanced wound care therapies with antimicrobial capabilities, that can be administered dependant on the patients wound. Antimicrobial dressings such as hydrogel, foam and alginate dressings are commonly used to treat advanced wounds. Some alternative antimicrobial dressings, which are also antimicrobial impregnated, include honey dressings, silver dressings and bioactive bacterial cellulose wound dressings. Due to widespread industry and social factors, such as a growing antimicrobial resistance epidemic, and an increase in chronic wounds, the need for manufacturers to explore alternative therapies is key.
Burn wounds are complicated to manage and so there is a need for continued rapid development of a wide range of advanced wound care therapies with varying compositions and indications. Pre-market testing for efficacy and safety, needs to match the diversity and complexity of novel wound care products entering the market.
Conclusions
Burn wounds constitute a significant healthcare challenge, with the potential for severe complications, notably infection, that can exacerbate patient morbidity and mortality. Burn injuries disrupt the skin’s natural barrier, rendering patients highly susceptible to microbial invasion. Infection in burn wounds primarily stems from endogenous skin flora, including Staphylococcus aureus and Pseudomonas aeruginosa, which can flourish in the compromised wound environment. The manufacturing of burn wound care products requires careful attention to materials, sterilisation, quality control, regulatory compliance, and product design. By addressing these considerations systematically, manufacturers can successfully bring innovative solutions to the burn care market while ensuring patient safety and product quality