In a UK population-based cohort study the mortality rate relating to the development of a diabetic foot ulcer (DFU) was 5% within 12 months and 42% within 5 years (Walsh et al, 2016). Treating DFUs and amputations is time-consuming, challenging and costs the NHS an estimated £962 million a year, with 90% of this expenditure related to ulceration (Kerr et al, 2019).
Development of a DFU can lead to polymicrobial colonisation that may progress, usually through the site of injury or ulceration, to affect soft tissue, tendons, joints and bone. Infection is commonly complicated by pre-comorbid conditions, such as peripheral neuropathy and ischaemia.
A compromised vascular supply and peripheral neuropathy are related to risk of initial trauma to the skin, infection, asymptomatic leucocytosis, impaired healing and optimal antibiotic absorption (Lipsky et al, 2016). Other influencing comorbidities that are taken into consideration when treating DFUs are renal, hepatic and multiple metabolic disorders.
Optimal treatment for a diabetic foot infection requires surgical debridement of the infected site, biopsies for microbial culture, with empiric and then targeted parenteral or oral antibiotic therapy. The most common isolated organisms causing soft tissue infection and osteomyelitis in acute DFUs are gram-positive cocci, namely Staphylococcus aureus and Streptococcus (groups A and B, sometimes C or G). Chronic infections may include Gram-negative organisms and anaerobic infections, particularly in ischaemic foot ulcers. It is unclear if these Gram-negative organisms are the causative destructive pathogens or colonising bacteria in these environments (Rao and Lipsky, 2007).
Unfortunately, polymicrobial culture results can make it difficult to target antimicrobial therapy and it is often necessary to use prolonged broad-spectrum therapy. In some cases, this can ultimately lead to development of multi-drug resistance and limited choices of available antibiotics for further treatment (Dang et al, 2003). Prolonged high doses of parenteral or oral antimicrobials are also known to be associated with serious systemic side-effects such as liver toxicity and renal impairment (Lipsky et al, 2016).
Calcium sulphate as an antibiotic delivery system
Gentamicin-loaded polymethylmethacrylate (PMMA) beads have been widely accepted as a local antibiotic delivery system for infected wounds. However, an intra-operative study by Neut et al (2001) retrieved PMMA beads from surgical sites that were found to be contaminated by gentamicin-resistant organisms. This was thought to be attributed to the beads’ surface acting as a biomaterial for bacterial colonisation as the antibiotic release declined over time.
One material with an advantage over PMMA is synthetic, medical-grade calcium sulphate. It is biodegradable and possesses predictable elution properties, osteoconductivity and an ability to fill bone and tissue cavities (Helgeson et al, 2009). In vitro studies have shown calcium sulphate impregnated with antibiotics such as vancomycin, gentamicin, amikacin, daptomycin and fusidic acid, delivers 200 times the minimal inhibitory concentration (MIC) levels for staphylococci over a minimum of 14 days, with non-detectable blood serum levels of antibiotic or calcium sulphate (Panagopoulos et al, 2008; Kanellakopoulou et al, 2009). Rauschmann et al (2005) also reported favourable in vitro results with much higher MICs of vancomycin and gentamicin being effectively released within the first five days. Armstrong et al (2003) argued that calcium sulphate impregnated with antibiotics maintained their antimicrobial characteristics for up to 120 days. However, this was an in vitro study and was limited to only vancomycin and tobramycin.
Local antimicrobial delivery systems have been widely used in DFU infections as a viable alternative to oral or parenteral antibiotics, or when required in combination with oral antibiotics (Agarwal and Healey, 2014), with significantly reduced systemic levels of toxicity (Maale et al, 2020).
Clinical studies have reported encouraging outcomes after surgical removal of devitalised tissue and bone followed by application of calcium sulphate beads impregnated with vancomycin and gentamicin into the wound space.
In a study by Gauland (2011), 279 out of 323 (86%) wounds healed without use of parenteral antibiotic therapy, 24/323 (7%) healed but required IV antibiotics as adjunct to antibiotic-impregnated STIMULAN, and only 20/323 (6%) went on to have minor or major amputations. The author considered this to be not insignificant. Microbiology biopsy sample results were not always available prior to application of the vancomycin and gentamicin beads, yet healing was still observed even if the culture results subsequently showed that organisms were not sensitive to vancomycin or gentamicin (Gauland, 2011). Vancomycin and gentamicin were chosen for their broad spectrum Gram-positive and Gram-negative microbial cover (Lipsky et al, 2016).
In a retrospective comparative study Qin et al (2019) looked at 48 patients with infected DFUs who were treated by either surgical debridement, application of vancomycin and/or gentamicin beads, plus intravenous antibiotics, or surgical debridement and intravenous antibiotics only. The results found no statistical difference in healing rates for osteomyelitis. The authors recognised that this may have been due to low subject numbers and that follow-up durations in both groups may not have been enough to show the outcomes of all the patients that may have influenced the recurrence of infection, healing or amputation rates. Another observation in this study was that both groups received IV antibiotics in addition to local antibiotic beads, suggesting that this was not a head-to-head comparative review and may not have changed any clinical outcomes.
More recently, Morley et al (2021) conducted a cohort study that retrospectively reviewed 137 cases treated with calcium sulphate loaded with vancomycin and gentamicin in foot ulcers with osteomyelitis (n=127) and soft tissue infection (n=10). Infection resolved in 88.3% of patients and 82.5% had an average wound healing rate of 11.4 weeks. In cases of peripheral vascular disease (PVD) and diabetes, time to wound healing was slightly longer.
Patients and methods
This clinical review looks at 26 individual, non-randomised cases and monitors outcomes of DFU response when antibiotic-loaded calcium sulphate beads were packed into wound cavities or dead spaces following primary surgical debridement. In each case, a conventional protocol was followed, osteomyelitis was radiologically confirmed, infected bone or tissue specimens were taken for microbiological culture to determine the causative microorganisms and antimicrobial sensitivities (Lipsky et al, 2016).
Patients with PVD had undergone vascular reconstruction to the affected limb prior to surgical resection of infected tissue and bone. Antibiotic-loaded calcium sulphate beads were mostly applied at the bedside 1–2 days post-surgery, although in two cases the application was conducted immediately following debridement in the theatre, based on the history of previous positive microbiological cultures.
Antibiotic addition guidelines were based on published literature (Laycock et al, 2018) using STIMULAN Rapid Cure® (Biocomposites, Keele, UK) and following the indication for use, contraindications and precautions.
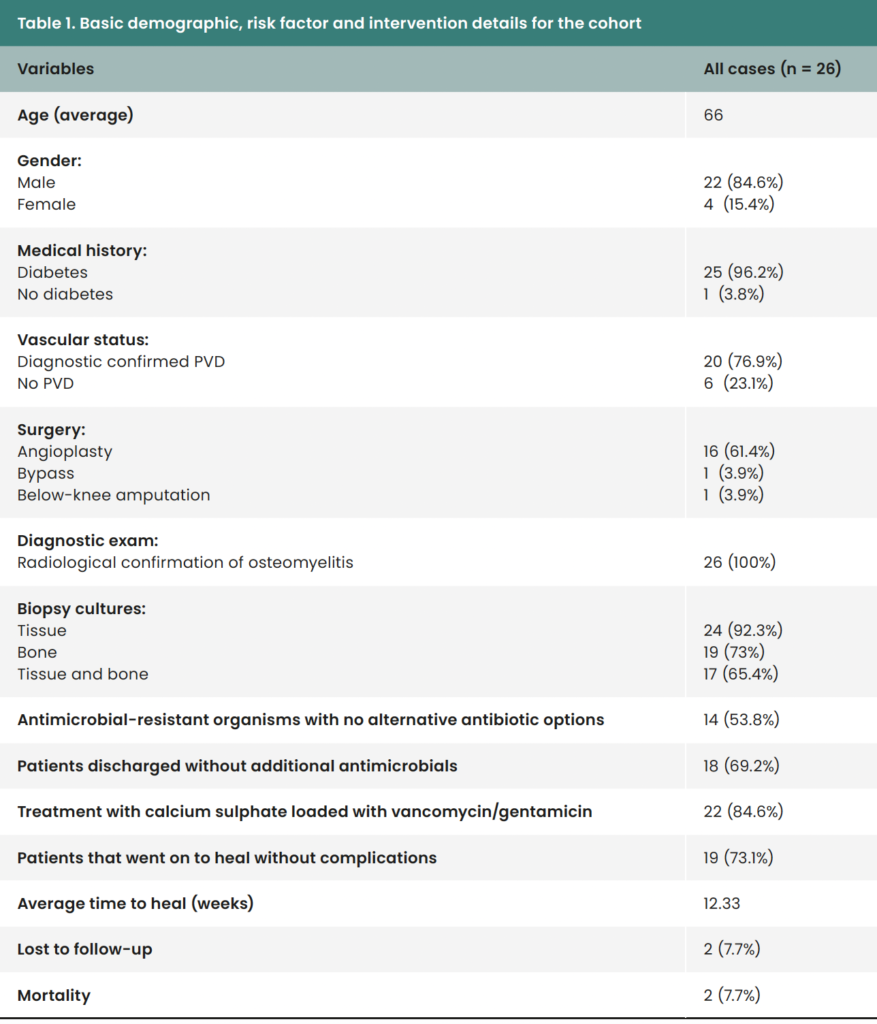
Patients were excluded from local antibiotic therapy where conventional targeted antimicrobials were the first choice of treatment and the surgical wound site could be treated with topical negative pressure (TNP). Patients were also excluded if they had untreated critical limb ischaemia or systemic sepsis related to a diabetic foot infection. Patient characteristics are shown in [Table 1].
Results and descriptive analysis
The decision to use STIMULAN as an antibiotic carrier was based on case-by-case discussion for the 26 patients, taking into account several contributing factors that prohibited the use of conventional antibiotic therapies. Influencing factors included growth of multi-resistant organisms, penicillin allergies where an oral/parenteral antibiotic was not an available option.
Multiple comorbidities and age were common contributing factors when considering antimicrobials. Other factors included thrombotic complication to an intravenous line insertion that prevented parenteral antibiotic therapy, cases of acquired Clostridium difficile infection on prolonged IV antibiotics, acute kidney injury resulting from prolonged intravenous antibiotics, a history of intolerance to quinolones and poor adherence to previous antibiotics or other regular medications.
One case involved ‘last-ditch’ limb salvaging by intra-wound antibiotic beads in combination with oral antibiotic therapy, in a patient with an extensive osteomyelitis of the calcaneum and mid-tarsal bones.
The choice of antimicrobials for impregnation was based on microbiological culture sensitivities. Patients received calcium sulphate with either monotherapy or a combination of antimicrobials: vancomycin, gentamicin, meropenem or amikacin.
Where indicated bone or deep tissue, in some cases both 17/26 (65.4%), were sent for microscopy, culture and sensitivities. Depending on the surgical debridement depth and size of wound either 5cc or 10cc calcium sulphate was mixed with the appropriate antibiotics (Laycock et al, 2018).
Following application of the antibiotic beads, patients that were medically stable were discharged on the same or next day and monitored closely in the outpatient foot clinic. Full blood profiles were taken on each review with added vancomycin, gentamicin and amikacin blood serum levels for the first 2 weeks. In each case the patients’ foot was offloaded appropriately and wound dressing management plans were written according to exudate levels.
The average time to wound healing was 12.3 weeks, with 19/26 (73.1%) of ulcers healed without the need for further surgical wound debridement. Patients were able to be discharged without oral or parenteral antimicrobials in 18/26 (69.2%) cases.
One patient did receive assisted wound closure with a TNP dressing to manage the excessive exudate levels. The TNP setting was used at a lower than usual wound healing therapy of 50mmHg continuous pressure.
One patient who had a below-knee amputation developed a stump wound dehiscence that was treated with antibiotic loaded beads applied to the stump wound cavity deep to the tibial bone.
The two patients that sadly passed away had complex underlying chronic health complications, and two patients were lost to follow-up.
Case examples
Case 1: clinical history
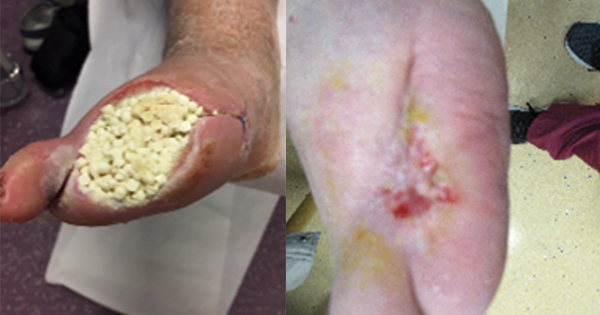
A 71-year-old man with a history of hypertension, PVD and type 2 diabetes was admitted for a pyogenic infection to the right hallux which resulted in amputation of the digit. He was discharged with a course of oral antibiotic therapy and followed up in the foot clinic. After several weeks of oral antimicrobials and weekly reviews, the patient presented to clinic with a tissue infection to the amputation site, which had spread to the second toe [Figure 1]. The multidiscliplinary team (MDT) agreed the wound had not responded to the current medical therapy. The patient consented for the second toe to be amputated. Proteus mirabilis and Pseudomonas aeruginosa were cultured from deep tissue and bone samples and found to be resistant to oral ciprofloxacin. Unfortunately, the patient discharged himself from hospital before a medical plan was completed and did not present to clinic for 2 weeks.
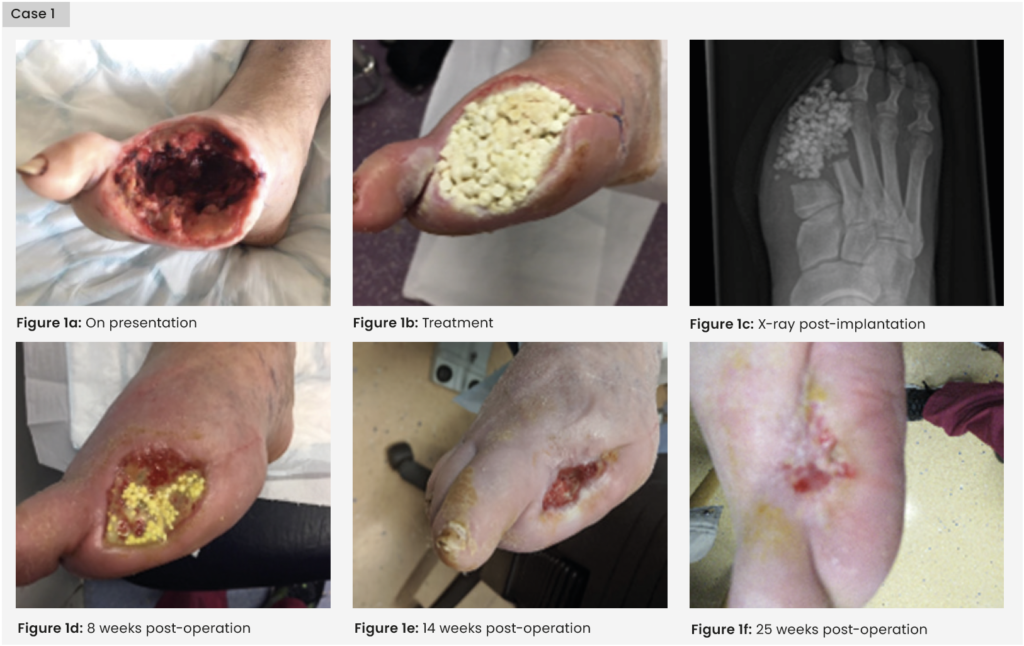
Treatment
The wound surface had a layer of biofilm, but was clinically stable with no tracking cellulitis or signs of systemic infection. The wound site was sharply debrided back to healthy bleeding granulating tissue by the podiatrist and 10cc calcium sulphate mixed with vancomycin and gentamicin was packed into the wound. The beads were sealed in with Adaptic Touch non-adhering silicone contact layer (3M) and Mefix tape (Mölnlycke). A secondary layer of sterile gauze, Soffban (BSN Medical) and crepe bandages were applied to manage the exudate levels. The antibiotic beads were left undisturbed to fully absorb into the tissue and bone. Oral and intravenous antibiotics were ceased.
Outcome
The wound healed well with healthy bone mineralisation and intact cortex seen on plain X-ray. During healing, the patient received a single short 7-day course of oral antibiotics for a suspected tissue infection at the same site. Healing was achieved at 25 weeks post intervention.
Case 2: Clinical history
A 39-year-old woman with a history of type 2 diabetes, peripheral neuropathy and previous right second and third toe amputations presented to clinic with inflammation and ulceration and a sinus to the apex of the right hallux [Figure 2]. An X-ray showed bone destruction and osteomyelitis that was not salvageable. The patient consented to a right hallux amputation and commenced 2 weeks of IV antibiotics followed by oral antibiotics on discharge. She was reviewed in the foot clinic weekly. Five weeks post-discharge, the patient was readmitted with an acute tracking cellulitis at the amputation site, which was still probing to bone. An MRI confirmed osteomyelitis in the first metatarsal and the patient had surgical revision of the infected bone. Microbiology results found Corynebacterium striatum in bone and tissue, which was resistant to penicillin, gentamicin, erythromycin and tetracycline. The MDT agreed a plan to discharge the patient with at-home parenteral antibiotics via a peripherally inserted central catheter (PICC) line. A complication developed with the PICC line with formation of a brachial to subclavian vein thrombus and it was removed.
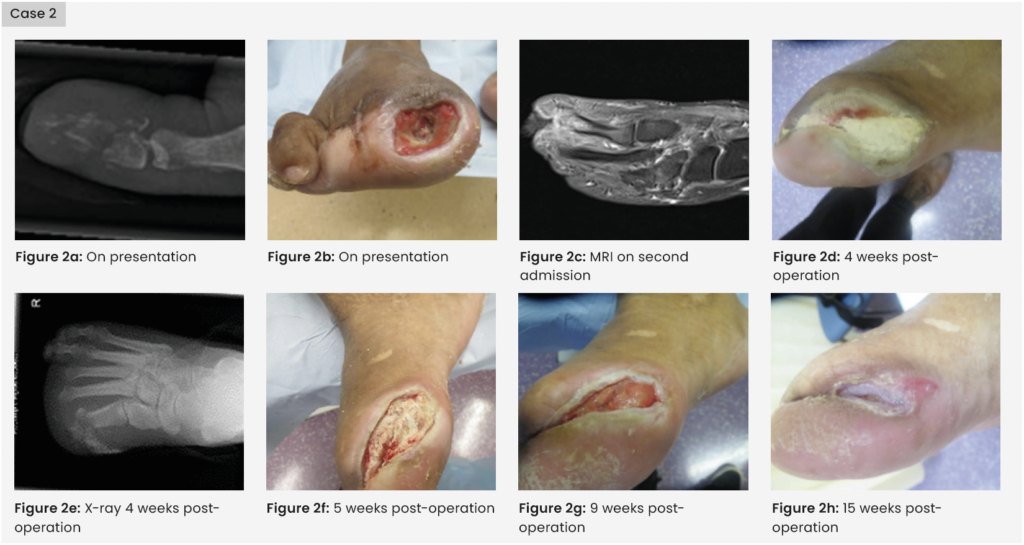
Treatment
The wound was packed with 5cc calcium sulphate mixed with vancomycin and gentamicin. The beads were sealed in with Adaptic Touch and Mefix tape. A secondary layer of sterile gauze, Soffban and crepe bandages were applied to manage the exudate levels. The gentamicin level at week one was <0.55mg/1 and week four was <0.40mg/1. At 5 weeks post implantation, the beads were removed due to suspicion of infection but the wound was then repacked with no further concerns.
Outcome
The wound had healed fully by 25 weeks, an excellent outcome in a patient for whom outpatient parenteral antibiotics were not an option.
Case 3: Clinical history
A 64-year-old man was admitted with a history of type 2 diabetes, PVD, hypothyroidism and chronic obstructive pulmonary disease. He had a left superficial femoral artery (SFA) angioplasty and stent 2 years previously. He had been scheduled for a left-sided downstream SFA/popliteal artery intervention; however, at the time his foot was stable, and this was cancelled due to the COVID-19 pandemic. The patient was admitted with chronic limb-threatening Ischemia (CLTI) of the left foot, and necrotic left first and second toes, extending to the dorsum of the foot [Figure 3]. He also had worsening pain at rest.
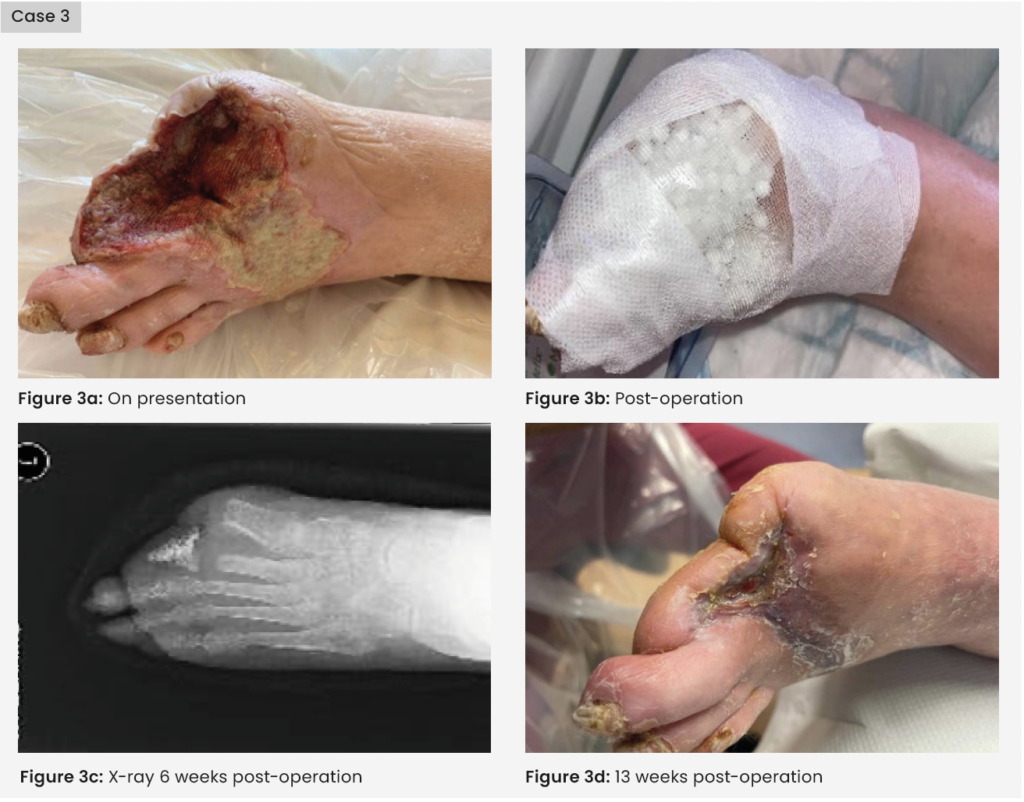
Treatment
A left leg SFA to posterior tibial artery (PTA) reversed native vein bypass was carried out, followed by first to second toe amputation on the left foot with dorsal necrotic tissue debrided. TNP dressing was applied post-op, but the patient found this very painful, so this was removed. Deep bone samples grew Enterobacter cloacae sensitive to co-trimoxazole, gentamicin, amikacin, meropenem and ciprofloxacin. 10cc calcium sulphate beads mixed with vancomycin and gentamicin were packed into the wound cavity and onto the dorsum of the thick biofilm wound layer. The beads were sealed in with Adaptic Touch non-adhering silicone contact layer, and Mefix tape. A secondary layer of sterile gauze, Soffban and crepe bandages were applied to manage the exudate levels.
Outcome
The 6-week X-ray showed healthy bone mineralisation and the bone cortex was intact. At 13 weeks, the wound had completely healed, a rapid time frame considering the severity of the initial wound. Post-healing, the patient had an uneventful angioplasty for a stenosis in the bypass graft to maintain arterial flow. Had these interventions not happened in a timely manner he would have most likely lost his leg.
Discussion
In all our cases treated with monotherapy or combination of vancomycin and gentamicin in calcium sulphate, targeted to the organisms cultured, wounds healed without complication. This may support the findings by Gauland et al (2011) that broad spectrum aminoglycosides offer a high concentration level of antimicrobial at wound level to combat infection and are favourable in healing. The average wound healing rates of 12.3 weeks is impressive and is comparable to rates of healing in Morley et al (2021).
The benefit of being able to apply local antimicrobial therapy meant that patients could receive high concentrations of therapy targeted at the site of infection, and could be discharged home the same day with a mitigated risk of systemic toxicity (Kanellakopoulou et al, 2009; Gauland, 2011). The usual patient pathways, either as inpatients receiving parenteral antibiotics, or waiting for a peripheral IV lines inserted for outpatient parenteral therapy resulted in delayed discharge adding excessive pressures on NHS clinical and financial resources. The impact of early hospital discharge has significantly benefited both the patient and hospital capacity pressures. Patients were followed up routinely in an acute clinic for close monitoring, with no added impact on outpatient clinics. In all cases no adverse complications to topical antimicrobial therapy were reported.
In one case, early discharge from hospital was facilitated using antibiotic beads and oral antibiotics at a time of bed capacity pressures during the COVID-19 pandemic. This also reduced the risk of nosocomial COVID-19 while the subject was an inpatient. In this particular case, targeted antibiotic beads were packed deep intra-wound, the foot off-loaded in an AirCast boot, TNP dressing applied to manage exudate levels and then safely discharged that day (COVID-19-free) and reviewed in the outpatient foot clinic.
The increased levels of exudate that have been noted from wounds packed with calcium sulphate beads have been described as yellow foamy fluid that subsequently reduces over approximately 2 weeks (Gauland et al, 2011). The theory for this high level of exudate includes pH changes of local antibiotic delivery and osmotic effects of the prism shaped calcium sulphate crystals on degradation, and possibly the impurities of the calcium sulphate itself (Robinson et al, 1999; Kelly et al, 2001 Lee et al, 2002). Lee et al (2002) examined the exudate from wounds packed with calcium sulphate beads and found that inflammatory cells were present.
Limitations include the small numbers and more robust empirical research is required to support the findings from this report.
Conclusion
Calcium sulphate loaded with antibiotics offers potentially promising outcomes and can sometimes serve as adjunct treatment for osteomyelitis in infected foot ulcers post-surgical removal of devitalised tissue and bone. An advantage of using a local antibiotic delivery system is the reduced risk of systemic toxicity, a high concentration of targeted antibiotic to treat tissue infection and osteomyelitis at a local level, the ability to fill dead wound space or cavities, and early safe discharge from hospital with local follow-up. The impact of this is a positive one for patients.
The results of the case studies presented show encouraging clinical results and potentially massive cost-saving benefits for treating tissue infection and osteomyelitis effectively in an out-patient setting and huge savings in bed days saved to the NHS. Based on the reported cases, it would be reasonable to suggest the following questions that local services can implement for best practice?
With the challenging emergence of global antimicrobial resistance, and effective ways to fighting infection in diabetic foot ulcers. What steps are required for topical antimicrobial therapy be applied by clinicians as a readily available, local, safe, effective adjunct tool to prevent amputations?
What are the potential economic benefits to the NHS if prompt delivery of topical antimicrobial therapy were readily available and used in community acquired foot infections?
Ethical statement
All data collected for this report will not be personally identifiable and kept confidential in accordance with the General Data Protection Regulations (GDPR) and Data Protection Act 2018. Patients provided verbal consent for case studies to be used in this clinical evaluation. Where indicated, patients gave verbal and written informed consent for photos to be used in publication. As this is a case review and local clinical evaluation in conjunction with standard patient care, ethics approval was not required.
Funding
No funding was received to support this article.