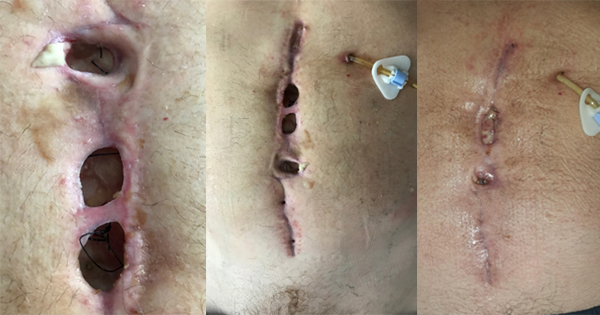
Aim: a clinical in market evaluation of 20 patients was carried out to assess the performance of the new ActivHeal Silicone Foam dressings in clinical practice. Study Design: The evaluation took place in two centres within the UK. The patients were treated according to product instructions and standard local practice. Data was collected at every dressing change until healing or the treatment of the product was discontinued. Results: Positive endpoints for exudate management, moist wound environment, periwound protection and patient satisfaction. By the end of the evaluation 20% (n=4) of the wounds had healed, 70% were improved and 10% (n=2) of the wounds may not have reduced in size but there had been a change in the condition of the wound bed with the removal of both necrotic and sloughy tissue. Conclusion: This study has shown ActivHeal Silicone Foam Border and Non-Border dressings to be an acceptable alternative to other silicone dressings in terms of patient comfort and clinician satisfaction.