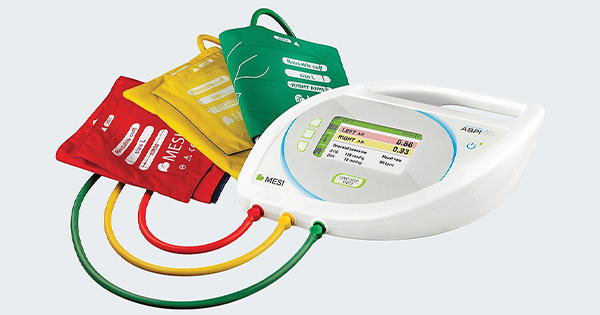
Ankle brachial pressure index (ABPI) is a non-invasive investigation used to assess for the presence of peripheral arterial disease (PAD), by comparing the systolic blood pressure in the ankle to that at the arm.
Compression therapy is the mainstay of treatment for venous leg ulcers (VLUs), but such therapy is contraindicated in the presence of PAD. The National Wound Care Strategy Programme (NWCSP) guidance on leg ulcers therefore recommends that every patient diagnosed with a leg ulcer should receive a full clinical assessment within 14 days, including an ABPI measurement, to exclude PAD before applying potentially harmful compression therapy (NWCSP, 2023). Identification of PAD in patients with a leg ulcers allows referral for appropriate treatment, and reduces the risk of sequelae, such as amputations or cardiovascular events.
ABPI can be measured manually using a sphygmomanometer and handheld Doppler ultrasound. However, performing manual ABPI can take up to an hour, and may be uncomfortable for the patient (NICE, 2023). Guidelines recommend patients take a period of supine rest of between 10 and 30 minutes before the measurement takes place, and blood pressure in six different arteries must be measured individually. The process also needs to be conducted by a healthcare professional with specialist training in using the equipment and performing the calculations; incorrect use may lead to poor diagnostic accuracy (Watson, 2022).
Limitations on staffing resources and time mean that in practice, ABPI measurements are not always conducted as directed by guidelines. A 2018 survey found that 40% of people with a leg ulcer did not have an ABPI assessment recorded (Gray, 2018). With an estimated 739,000 people in the UK living with leg ulcers (NWCSP, 2023), this amounts to a significant burden of people who may be receiving suboptimal or inappropriate care.
Automated ABPI measurement devices aim to relieve some of the burden of ABPI measurement. They can reduce the time needed to assess ABPI as multiple cuffs allow measurements to be taken in several limbs simultaneously, do not require a prolonged period of supine rest as the simultaneous measurements reduce the risk of inconsistency between consecutive cuff measurements, and may lead to more accurate results as calculations are performed automatically (NICE, 2022; Watson, 2022; Wounds UK, 2019).
NICE diagnostics guidance: automated ABPI measurement devices in people with leg ulcers (DG52)
NICE published guidance in May 2023 on the use of automated ABPI devices (NICE, 2023). This guidance did not recommend the routine use of automated ABPI devices to detect PAD in people with leg ulcers, and instead recommended that they are currently used only in the context of research [Table 1].
The guidance was based on health economic modelling, which determined that automated ABPI devices were unlikely to be cost-effective unless they had a positive effect on time to treatment, which was not clearly demonstrated by existing studies (NICE, 2023). It was also considered unclear whether automated ABPI measurements positively affected clinical outcomes. Most of the studies reviewed by the committee involved the use of automated ABPI measurement in people without leg ulcers, and therefore there was insufficient evidence of their accuracy in detecting PAD in people with leg ulcers (NICE, 2023).
Within the recommendations on further research, the guidance called out three areas where NICE felt additional data would be of particular use (NICE, 2023):
- Diagnostic accuracy in people with leg ulcers
- The impact of automated devices on time to treatment
- Clinical outcomes such as time to healing or incorrect use of compression.
MESI survey of healthcare providers
Dr Daphne Hazell (GP and Clinical Researcher) presented the results of an anonymised survey, sent by MESI (a manufacturer of automated ABPI devices) in September 2023 to all UK health services and centres using MESI ABPI devices. The survey aimed to evaluate wound care practitioners’ experiences and opinions on the use of the MESI automated ABPI device, and whether this had changed because of the NICE DG52 guidance. In response to the request from NICE for further research, the survey also collected data on resource use with automated versus manual ABPI measurement devices.
Responses were received from 207 clinicians. The majority of these (59%) were from primary care, and a further 20% were tissue viability nurses. The remaining respondents included community nurses and lymphoedema nurses [Figure 1]. Of the respondents, 66% had more than 5 years of experience in their current role.
All respondents were using a MESI automated ABPI device; 71% were using the MESI automated ABPI MD, 19% were using the MESI mTablet ABI, and 10% reported using both devices. More than two-thirds (68%) said they had been using the device for more than 12 months. Most respondents (88%) were trained and competent to perform manual ABPI assessments as well as using the automated device.
Results of the survey
Almost all respondents (93%) reported using the automated devices 0–3 times per day. Only 3 respondents used them more than 6 times per day; one service reported using it more than 12 times per day on average. The average reported time for ABPI assessment using an automated device was 10 minutes 56 seconds, compared with an average of 32 minutes for manual measurement [Figure 2]. Using the device was considered ‘easy’ by 67% of respondents, ‘normal’ by 20% and ‘difficult’ by 13%.
If the automated device was unable to measure the ABPI, 80% of respondents would perform a manual assessment using a handheld Doppler; the remaining 20% would refer the patient to tissue viability or vascular services for this assessment. If the device returned an ABPI value of 0.8 or less, indicating potential PAD, 52% would refer the patient directly to vascular services, 43% would perform a manual ABPI measurement to verify the results before referral, and 5% would refer to a tissue viability nurse for manual assessment before vascular referral.
A majority of respondents (80%) reported that their patients were able to access compression therapy faster since the introduction of the automated ABPI device. The median reduction in waiting time was 2–4 weeks. After introduction of the automated device, most patients were now meeting the NWCSP recommendation of receiving ABPI measurement within 2 weeks of their ulcer diagnosis. One-fifth of respondents said that they were now able to offer compression therapy to patients, when previously they were not.
How has the NICE guideline changed wound care professionals’ practice?
Survey respondents were asked whether they had changed their use of automated ABPI devices since the publication of the DG52 recommendations; the same question was also asked to Wounds UK annual conference attendees, who were able to respond throughout the duration of the conference using the Whova conference app on their mobile phones.
Among survey respondents, 56% said they had not changed their use since the NICE guidance; 21% answered ‘unknown’. In presenting these results, Dr Hazell commented on the unusual nature of the ‘unknown’ responses, since it was unexpected that respondents would feel unable to answer whether they had changed their practice. When those who answered ‘unknown’ were excluded from the results, the percentage of those who had not changed their practice increased to 72%.
Among conference attendees who reported that they had used automated ABPI devices before May 2023, 48% reported that they had not changed their practice at all as a result of the guideline publication. Another 36% reported some reduction in their use of automated machines, while only 16% said they had stopped using them altogether [Figure 3].
Respondents were also asked whether they had any concerns that the NICE guidance on automated ABPI devices was causing delays in patients accessing compression therapy. Excluding ‘unknown’ responses, 42% of survey respondents and 33% of conference attendee respondents said they thought this was the case [Figure 4].
Response from healthcare providers
Conference attendees expressed surprise that the accuracy of the automated devices had been called into question. With manual ABPI measurement, six different blood pressure measurements must be taken individually, and the time delays between measurements could potentially affect the results; attendees felt that the ability of the automated devices to take simultaneous measurements led to increased accuracy in most cases.
One nurse reported that the automated devices were widely used by the district nurses in her local area. Following the publication of the NICE guidance, the nursing team had conducted a risk assessment, which had concluded that the potential harm of delays to care caused by increased waiting times for manual Doppler ABPI assessment outweighed the risks of continuing to use the automated devices, and the informed decision had therefore been made to continue with automated ABPI assessment.
Some attendees also expressed their concerns that the NICE guideline, while evidence-based and possibly representing the gold standard of ideal care, did not reflect the realities of current practice. Wound care professionals reported that their clinics had adopted the automated ABPI devices in part to address service pressures and workforce shortages, and that they felt the guideline had not offered any practical advice on how this could be managed if the automated devices were no longer used. It was also perceived that tissue viability nurses and community nurses, who are among the most frequent conductors of ABPI assessments in clinical practice, were underrepresented on the guideline development committee.
Summary and conclusions
ABPI measurement is an important component of ensuring the correct care is provided to patients with leg ulcers, yet evidence shows it is underused in clinical practice (Gray, 2018). Automated ABPI technology offers the potential to help improve measurement accuracy and patients’ experiences of care, while also maximising the use of clinic resources and staff time. Whether conducted using manual or automatic devices, ABPI forms only one part of the assessment of a patient with a leg ulcer; a comprehensive review and the use of clinical judgement in assessing a patient’s vascular risk remain indispensable.
NICE guidance does not currently recommend the routine use of automated ABPI measurement devices, due to concerns about their accuracy in detecting PAD in people with leg ulcers, and uncertainty around whether the technology is associated with decreased time to therapy or improved clinical outcomes.
Nonetheless, the majority of wound care professionals questioned via this written survey and conference voting app had not changed their practice as a result of the guidance, 6 months after its publication. The results of the survey indicate that wound care professionals are able to perform ABPI assessments faster using automated devices compared with manual methods, and that this is linked to shorter waiting times for compression therapy. As well as the qualitative benefits seen, the survey also assessed healthcare professionals’ attitudes towards the new guidance, and found that a substantial portion feel it has the potential to negatively impact patient care.
The NICE guideline development process includes a comprehensive literature review, intended to comprehensively assess the evidence. Nonetheless, Dr Hazell remarked that this literature review often prioritises randomised controlled trials, which provide the highest grade of evidence, but do not necessarily reflect the realities of frontline clinical practice. This may contribute to the reluctance among the professionals surveyed to change their practice in a way that reflects the new guidance.
Dr Hazell ended the presentation by noting that to address the evidence gaps that NICE identified, involvement from device manufacturers would be essential, and issued a call to action to wound care nurses to collaborate with industry to support the collection of such real-world evidence. She finally encouraged all conference attendees to engage with the NICE guideline development process wherever possible, to help ensure that future appraisals are able to take full consideration of clinicians’ experience and expertise in providing hands-on patient care.