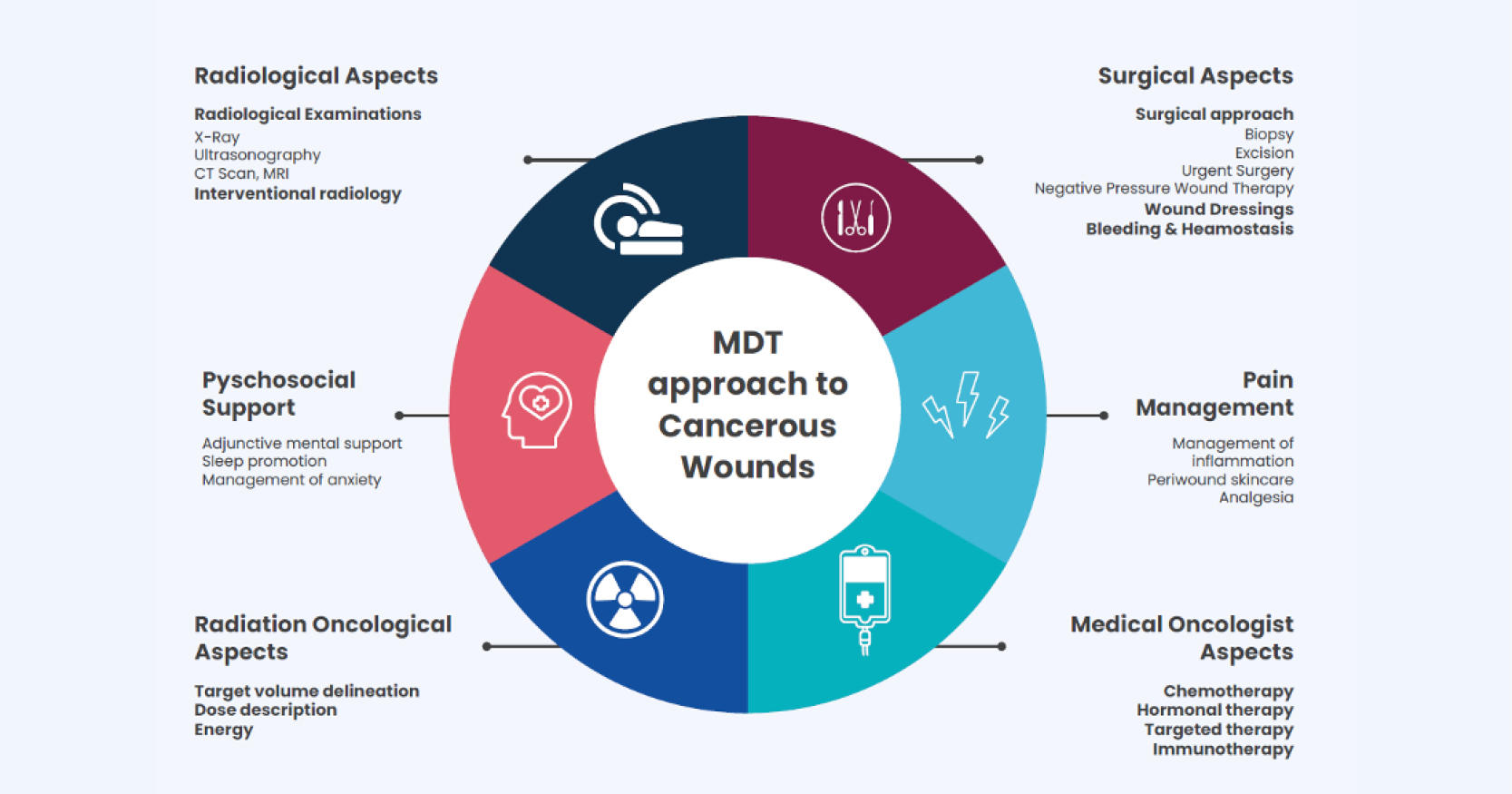
Necrotising fasciitis is a severe and life-threatening soft tissue subcutaneous skin infection; it can extend into the deep fascia but not into underlying muscle (British Medical Journal, 2021) and is commonly referred to as the “flesh-eating disease” (NHS, 2019). Although uncommon, necrotising fasciitis is not rare, it can occur because of a minor injury or postoperatively (The Lee Spark NF Foundation, 2020). Nawijn et al (2021) reports there are few studies on the incidence of necrotising fasciitis worldwide, it is estimated that within the UK, 500 people annually develop the infection (British Medical Journal, 2021). Studies in the Netherlands estimate the mortality rate for the disease is 23–29% and surgical amputation occurring in 11–14% of cases (Nawijn et al, 2021). Necrotising fasciitis commonly occurs in the lower limbs, perineal region, genitals and abdomen (Misiakos and Bagias, 2017).
Early symptoms of necrotising fasciitis can include localised pain, swelling and erythema (Misiakos and Bagias, 2017). Skin changes have been reported including: crepitus (popping/clicking), necrosis and haemorrhagic blisters on the skin filled with blood caused by damaged blood vessels (cutaneous blisters), alongside these symptoms patients can experience fever, hypotension and tachycardia (Misiakos and Bagias, 2017). Early diagnosis, preferably within four hours, and surgical management are integral in reducing the spread of this severe infection, while delays in treatment, of more than 12 hours can be fatal (Misiakos and Bagias, 2017).
Case Study
A 51-year-old female with a raised body mass index (BMI), a weight of approximately 70kg, was admitted into accident and emergency with a large area of necrosis across the abdomen. She was extremely unwell within the emergency department and experienced of swelling, severe pain and erythema across the abdomen, she had no significant past medical history. The patient was taken to theatre for debridement, and tissue samples from the abdominal wall were sent for histology, the appearances of which were consistent with necrotising fasciitis. An X-ray showed a perforated sigmoid causing abscess, which leaked into the abdominal wall tissue causing necrotising fasciitis. As a result of these diagnostics, she was transferred from the accident and emergency department for emergency surgery.
The patient was in theatre for several hours where she required a laparotomy, by performing an incision into the abdominal cavity, and Hartmann’s procedure — this involves removal of the sigmoid colon and upper rectum —an end colostomy is then formed. Due to the complexity of the surgery and extensive tissue removal, she was admitted to the intensive care unit and was ventilated.
Tissue viability referral for advice and clinical support was requested two weeks postoperatively due to recurrent surgical interventions required to stabilise her condition. In total, she attended theatre on six occasions for wound debridement and washouts. Misiakos and Bagias (2017) suggest further debridement surgery is usually required with necrotising fasciitis until healthy tissue is found and there is no further evidence of the spread of infection. Wound debridement in necrotising fasciitis cases is extensive to ensure that all necrotic and infected tissue is removed, and are as wide as the rim of the cellulitis (Duan et all, 2020).
On initial tissue viability assessment, the patient had extensive lower abdominal tissue loss extending to the groin (Figure 1–3). She had a newly formed stoma, which was initially assessed by a stoma clinical nurse specialist in collaboration with Tissue Viability Nurses. As a result of extensive tissue removal, the patient was referred to the plastic surgery department, but neither the wound nor the patient were stable at this point.
The wound was assessed and found to be stable and appropriate for the use of negative pressure wound therapy (NPWT). Li et al (2015) say the timing for wound closure is essential, if the wound is closed to early there is a risk of residual infection and poor healing. NPWT is an effective method for management of necrotising fasciitis as it removes infected material from the wound bed that can hinder tissue healing, (Sammoni et al, 2012). Negative pressure creates the ideal wound healing environment, by increasing granulation and local blood perfusion (Li et al, 2015).
Challenges
There were numerous challenges faced when managing this extensive wound. The wound measured 40cm in length by 20cm in width, and there were areas of undermining, which is where the destruction of tissue that surrounds the wound margins causes deep pockets; recognising these areas is important in providing the best management in the wound healing process (Brown, 2013).
Wound bed preparation involved irrigating the wound with a polyhexamethylene biguanide (PHMB) solution. The solution moistens the wound bed, aids in the removal of any dressing debris or residue, and can reduce bacterial bioburden (Moore et al, 2016). The periwound area was extensively excoriated due to the high exudate levels from the wound and stoma (Figure 2).
The enzymatic activity of proteases within wound exudate impairs the barrier function of the skin, leading to maceration and or excoriation (Vowden et al, 2015; Figure 2). In an attempt to reduce excoriation, a film barrier was applied using an applicator to protect the wound edge, then strips of a thin hydrocolloid (a breathable, transparent adhesive dressing) were sited around the wound edge to protect against further excoriation (Figure 4). This allowed for an ideal waterproof border, which reduced friction, and aided the adhesive application of the clear film outer dressing.
The wound bed was protected with a non-adhesive contact layer, and then packed with an antimicrobial gauze filler. Gauze was chosen over the alternative foam dressing as it can conform, and fill the undermining areas, while also building up the wound bed. The gauze, in conjunction with the wound contact layer, is non-adherent and can be well tolerated in painful wounds (Patton et al, 2013). Multiple pieces of gauze filler were used to build up the wound bed above surface level, ensuring that the dressing is level with the surface of the skin when vacuum therapy is applied. It is important at this point to document how many gauze pieces are inserted, so the same amount can be counted out on next application of the dressing to avoid any retention of the product (Zaver and Kankanalu, 2022).
The stoma was sited very close to the wound edge and there was challenges with this; with the unpredictable action of the stoma, there was a risk of faecal contamination with intestinal bacteria into the wound. Faecal leakage can be a common cause of negative pressure leaks (Zhang et al, 2020) thus making the therapy ineffective. To achieve a good seal, hydrocolloid strips were placed around the stoma and around the wound edge, this created a border to apply the clear drape.
Transparent adhesive drape cut into strips are required to ensure the area is airtight (Zaver and Kankanalu, 2022). The drape was applied over the wound area. A small 10 pence piece size hole was cut into the drape; this allows the soft port to be applied. However, due to the large wound area 2 ports were required to ensure optimum therapy and vacuum, as well as allowing the removal of exudate. The 2 ports were connected via a Y connector to the NPWT device and sub-atmospheric pressure of 80mmHg was applied (Figure 5).
Sub-atmospheric pressures aid in the reduction of wound space, promotes tissue tension and aids cell regeneration from the wound base (Zavier and Kankanalu, 2022). Once a seal was achieved, additional hydrocolloid WAS applied around the stoma to build it up (Figure 6); this allowed for a good surface for the stoma bag to be applied (Figure 7) and separated the stoma from the wound.
High exudate levels from the wound were well managed well using the negative pressure collection canisters; These were changed twice a week or when they became full. Due to the extracellular fluid loss, dietitians were involved to provide nutritional support. The patient required supplementary intravenous fluids to reduce the risk of secondary dehydration; this is supported by the recommendations of Zaver and Kankanalu (2022) .
The dressing required changing 2–3 times per week. Due to a lack of experience from ward based nurses, reduced staff, time constraints, and the complexity of the wound, the dressing application was undertaken by experienced Tissue Viability clinical nurse specialists and took between 2–3 hours. Therapy should only be applied by those who are competent in the use of this technique with the appropriate knowledge and clinical skills.
A holistic patient assessment must be undertaken to determine whether they are appropriate for NPWT (PA Patient Safety Authority, 2014). Policies and protocols for each facility should be followed to ensure the correct use of NPWT.
The patient spent one month in intensive care before being transferred to a general surgical ward for a further two weeks, and later transferred to the plastics ward. On transfer to the plastics team the wound displayed granulating tissue across the wound bed and had reduced the wound had reduced in size; the surrounding skin was much improved (Figure 8–10).
The patient continued NPWT when she was discharged home from plastics. Community nurses, with support from district nurses continued the care and the patients wound healed fully but had extensive scar tissue.
Conclusion
Necrotising fasciitis is a relatively unknown disease;
It’s destruction is quick and increases mortality and morbidity rates. Early and accurate diagnosis is integral in the management of necrotising fasciitis and can significantly reduce the mortality rate. It is therefore important for clinicians and surgeons to increase awareness to recognise early skin changes in order to perform prompt, early debridement, along with appropriate antibiotic therapy. Using NPWT ensured the wound was in the ideal environment for optimum healing. A multidisciplinary team approach is integral in the successful treatment of necrotising fasciitis