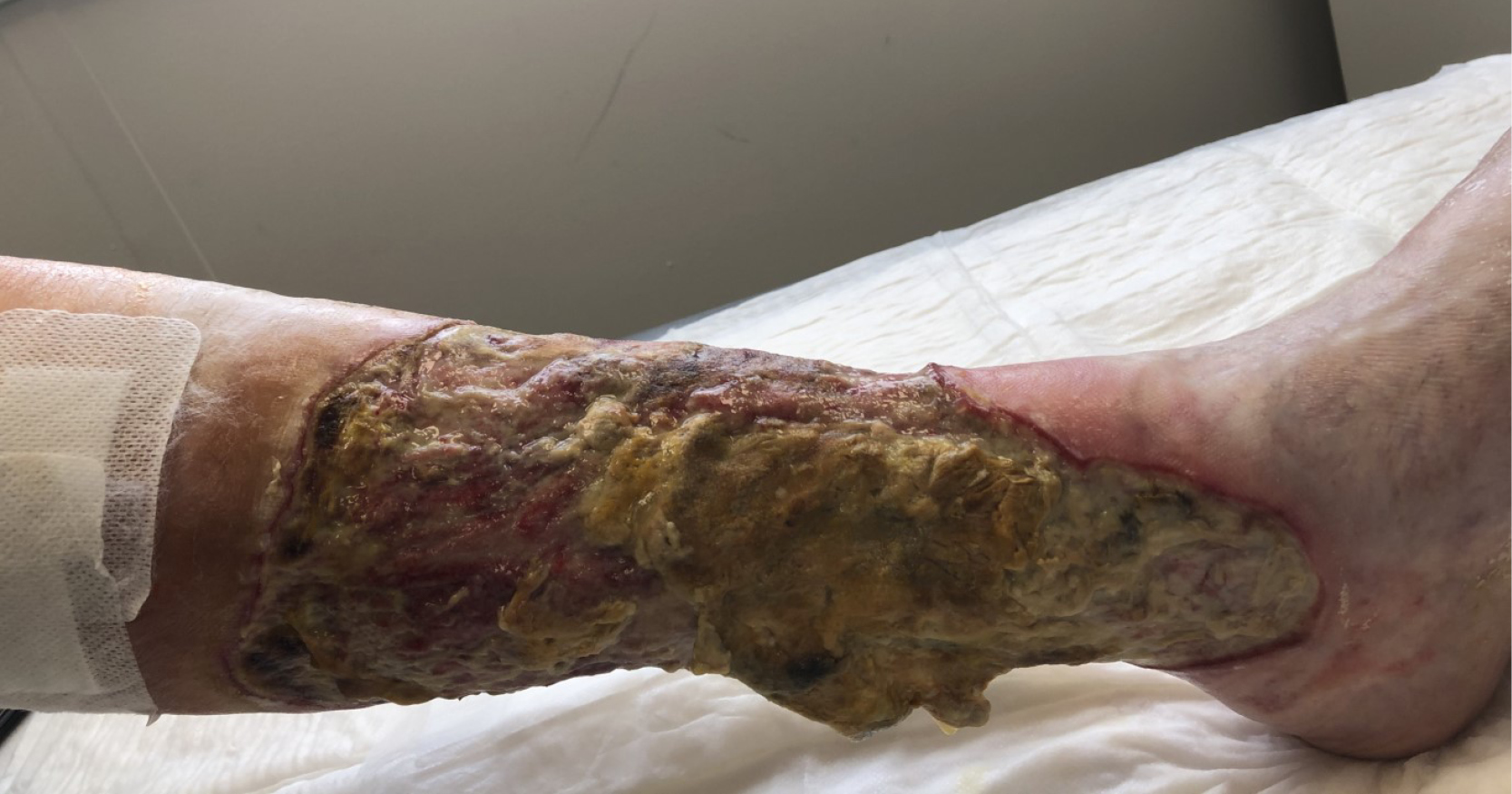
Hard-to-heal wounds present a major challenge for healthcare providers globally and approximately 2–6% of the population worldwide is affected (Järbrink, 2017). Many factors are shown to influence the wound healing pathway, one being the bioburden of the wound that represents a significant impediment to normal healing (Metcalf et al, 2013). A majority of hard-to-heal wounds contain biofilm (Malone et al, 2017) and it is widely recognised that almost all chronic infections are due to biofilm microorganisms (del Pozo et al, 2007; Høiby et al, 2014; WUWHS, 2016). Once microorganisms colonise the wound, they can form a matrix of extracellular polymeric substances (Høiby 2014), which act as a barrier to both host defenses (Wolcott 2008) and external antimicrobial treatments (del Pozo et al, 2007). As such, wound biofilm presents an infection risk and barrier to timely healing. Further complicating the treatment of hard-to-heal wounds is the occurrence of antibiotic-resistant microorganisms such as methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Pseudomonas aeruginosa (RPA; Center for Disease Control and Prevention, 2019).
To improve the management of hard-to-heal wounds, it is necessary to address tenacious biofilm. Biofilm must be disrupted and reduced or removed to initiate and support healing, recently described as part of Wound Hygiene (Murphy et al, 2020), and to prevent and treat local infections (Bjarnsholt, 2013). Physical debridement has been reported to be an effective way to prevent the persistence and regrowth of biofilm and make previously protected microorganisms susceptible to standard antimicrobial treatment (Wolcott et al, 2010). Various methods of wound debridement are used to remove devitalised tissues (e.g., necrotic tissue, slough, fibrin) from wounds, but the most effective debridement techniques, such as surgical, sharp and curettage, require specialist training so are not routinely available as standard of care. An ideal debridement methodology would therefore enable the removal of both unwanted devitalised host tissues and biofilm in a simple, safe and effective way that can be conducted without specialist training. While there are products and methodologies available for tissue debridement or biofilm management, until recently, easy-to-use debridement products with both properties have been lacking.
The purpose of this article is to examine the antibiofilm effect of ChloraSolv® Wound Debridement Gel, a novel innovative technology for debridement and wound bed preparation of hard-to-heal lower extremity wounds. ChloraSolv is an amino acid-buffered hypochlorite gel that facilitates the removal of devitalised tissue without the need for surgical or sharp debridement (Bergqvist et al 2016; Eliasson et al 2021; Atkin et al 2022), combined with demonstrated antimicrobial activity (Eliasson et al 2021). In this study, a series of increasingly challenging and clinically relevant laboratory test methods have been used to examine the performance of ChloraSolv against biofilm. The performance of this new wound debridement gel was also assessed in comparison to other wound debridement techniques/products available to healthcare professionals.
METHODS
Minimum biofilm eradication concentration (MBEC) adapted assay
The antibiofilm activities of ChloraSolv (RLS Global, Sweden) and Prontosan Wound Irrigation Solution (B. Braun, Germany; often used as an antimicrobial wound soak) were evaluated using previously developed methods. The standard biofilm susceptibility method, ASTM E2799-17 Minimum Biofilm Eradication Concentration (MBEC) assay for susceptibility of P. aeruginosa), which is also used for testing S. aureus biofilm (Ceri et al, 1999), was adapted to be made more stringent for this study. The main differences: biofilm was cultured on the bottom and side of the wells of 96-well plates, S. aureus (ATCC 29213) biofilm was cultured on round-bottomed plates (Costar; Coring Inc., Kennebunk, US) and P. aeruginosa (ATCC 27853) biofilm was cultured on flat-bottomed plates (Greiner Bio-One, Frickenhausen, Germany) as preferred by the microorganisms (Liu et al, 2020), and a biofilm culture time of 48 hours
Overnight cultures of the challenge microorganisms, S. aureus or P. aeruginosa, were prepared in Todd Hewitt broth (BD Biosciences, USA). The overnight culture was re-cultured in fresh broth and was allowed to grow until logarithmic phase. The resulting bacterial pellet was diluted to a 1–2×109 colony-forming units (CFU)/mL stock solution. The stock solution for each challenge organism was further diluted to approximately 1×105 CFU/mL. Stock solutions were added in 5µL volumes to 100µL of the relevant media within the wells: 0.5% Tryptone Soy Broth (TSB; BD Biosciences, USA) with 0.2% glucose or biofilm minimal media (M63; VWR, UK) with 0.2% casamino acids, 0.2% glucose and 1mM magnesium sulphate, respectively. Plates were sealed and biofilm was cultured at 37°C for 48 hours.
Before biofilm treatment, planktonic and loosely adhered cells were firstly removed by washing twice in 100µL volumes of phosphate-buffered saline (PBS; Medicago, Sweden). Premixed ChloraSolv and Prontosan were added to the wells in 100µL volumes. The treatments remained on the biofilm within the wells for 0.5, 2, 5 or 15 minutes. Following these time points, to neutralise antimicrobial effects of ChloraSolv, 100µL of neutralising agent (1.096g di-sodium hydrogen phosphate dihydrate, 0.208g sodium dihydrogen phosphate dihydrate, 0.5g sodium thiosulfate pentahydrate, 3.0g Tween 80, 0.3g egg lecithin, and 0.1g L-histidine monohydrochloride monohydrate in 100mL water) was added to each well. Prontosan’s antimicrobial effects were neutralised by removing the solution and performing repeated washing with 100µL volumes of PBS. Biofilm was then disrupted by scraping the wells using a sterile pipette tip, as described in the literature (Lemos et al, 2010). Serial dilutions on the resultant suspensions were performed to enable enumeration on Bacto agar (Saveen Werner, Sweden) in CFU/mL. Two-sample t-tests were conducted using Microsoft Excel for statistical comparison of bacterial counts for ChloraSolv versus Prontosan where p<0.05 was considered a statistically significant difference.
Biofilm live-dead staining
The adapted MBEC assay was also used for the assessment of the effects of ChloraSolv on P. aeruginosa and S. aureus biofilm using fluorescence microscopy. Viability of the treated biofilms was visualised using the BacLight Bacterial Viability Kit (Invitrogen, Molecular Probes, Carlsbad, USA) following treatment with ChloraSolv for 0.5, 2, 5 and 15 minutes. A 10µL sample was aspirated from the treated biofilms by pipette and 50µL of LIVE/DEAD solution (50µL, 2.5:1000 of component A, SYTO 9 green-fluorescent nucleic acid stain, and 2.5:1000 of component B, red-fluorescent nucleic acid stain propidium iodide diluted in PBS) was added to the samples in centrifuge tubes. Samples were incubated for 15 minutes at room temperature in the dark then centrifuged for 5 minutes at 14,000 rpm. A 50µL volume of solution was removed from the tubes and the remaining 10µL containing the treated cells were resuspended by briefly vortexing. A 5µL droplet was then mounted on a microscope slide (Thermo Fisher, Waltham, USA), and the stained bacterial samples were examined using a Zeiss AxioScope A.1 fluorescence microscope. To enable easier viewing within this article, all images were automatically processed with ‘Brightness + 40%’ in Paint 3D image viewing software.
Gauze biofilm model
The antibiofilm performance of antimicrobial solution soaks and debridement pads, cloths and gels, was assessed using an adapted gauze biofilm model that had previously been used to assess the antibiofilm performance of wound dressings (Bowler and Parsons, 2016). The test method was subsequently validated by a BSI and UKAS accredited laboratory (Perfectus Biomed, now part of NAMSA, Daresbury, UK) for use with methicillin-resistant Staphylococcus aureus (MRSA) ATCC BAA-1556 and multidrug resistant P. aeruginosa NCTC 13437 (VIM-10 metallo-carbapenemase, VEB-1 ESBL) (RPA) with respect to repeatability, robustness, repeatability-precision and ruggedness. Before conducting testing, the ability of Dey-Engley Neutralizing Broth (DENB; Acumedia, Lansing, USA) to neutralise all test products was demonstrated (Table 1).
Colonies of the challenge microorganisms were separately dispersed in Maximum Recovery Diluent (Acumedia, Lansing, USA) to obtain approximately 1×108 CFU/mL. The bacterial suspension was added in 500µL volumes to 49.5mL of 50:50 TSB (Lab M, UK): Fetal Bovine Serum (Gibco, France) in wide-mouth/neck Durans (SLS, UK; these containers have a wider opening and width compared with standard Durans, enabling the N-A gauze to lie flat on the bottom). A N-A Gauze sterile dressing (9.5 x 9.5cm; 3M/KCI/Acelity, UK) was placed flat in the bottom of each Duran. The Durans were incubated in a shaking incubator at 50rpm at 35 ± 3°C for 48 hours to allow mature biofilm to develop on the gauze.
The biofilm-colonised gauze was removed from the growth medium and added to 300mL 0.85% saline (Oxoid, UK) within a sterile stomacher bag. The gauze was rotated 5 times clockwise, 5 times anti-clockwise, squeezed 10 times with one hand, then left to stand for 2 minutes, to ensure that planktonic or loosely adhered cells were removed. The gauze was transferred to the lid of a 55mm sterile contact plate, to enable the treatment area to be focused within a 30mm diameter central circle.
Prontosan (B. Braun, Germany), Octenilin (Schülke & Mayr, Germany), Veriforte (Veriforte, Austria) and Granudacyn (Mölnlycke, Sweden) (wound irrigation solutions) were tested as antimicrobial solution soaks, whereby 10mL of antimicrobial solution was added to sterile Topper 8 Gauze Swabs (Acelity, UK) (2 ply of 5 ply pack was used), which were placed onto the biofilm-colonised gauze for 15 minutes. The swab soak was then removed, and the back of the gauze swab piece was used to wipe the biofilm in one direction 5 times by hand, focussing on the central 30mm diameter area. A 20mL volume of the same irrigation solution was used to irrigate the area using a large sterile syringe (BD Plastipak, Spain), focussing on the central 30mm diameter area.
Of the physical debridement devices that were moistened, Debrisoft (L&R Medical, UK) was moistened with 20mL of 0.85% sterile saline, Prontosan Pad (B. Braun, Germany) was moistened with 20mL Prontosan Wound Irrigation Solution (B. Braun, Germany), while UCS wipes (Medi, UK) were not (they are provided pre-moistened). Operators applied gentle pressure with the pads/wipes, performing small circular motions for 1 minute, then up-down and left-right strokes for 1 minute, focussing on the central 30mm diameter area. A 20mL volume of saline for the Debrisoft test and 20mL volume of Prontosan Wound Irrigation Solution for the Prontosan Pad test was used to irrigate the using a large sterile syringe, focussing on the central 30mm diameter area.
ChloraSolv was applied to the biofilm-colonised gauze according to the manufacturer’s instructions by dispensing half of the syringe (1.5mL) directly to the central 30mm diameter area and left for 2 minutes. A Topper 8 Gauze Swab moistened with 10mL saline was used to remove the ChloraSolv gel from the biofilm. The same swab was folded in half (with the used gel inside) and the back of the gauze swab piece was used to wipe the biofilm in one direction 5 times, focussing on the central 30mm diameter area. This process was then repeated for the other half of the syringe contents.
Following treatments, the biofilm-colonised gauze was transferred to a sterile cutting mat and a 24mm diameter circle, within the 30mm diameter treatment area, was cut using a sterile 24mm diameter biopsy punch. The 24mm gauze circles were stomached on high for 4 minutes in 30mL DENB as a biofilm removal and neutralisation step. Viable CFU were enumerated on Tryptone Soy Agar (Acumedia, Lansing, USA). Two-sample t-tests (unequal variance applied to the data) were conducted for statistical comparison of data using Microsoft Excel.
No-treatment biofilm controls were enumerated to indicate initial biofilm bacteria. Negative controls for tested debridement techniques/products replaced application of any antimicrobial solutions or gels, with the same volume of 0.85% saline. All controls were performed in triplicate.
RESULTS
Minimum biofilm eradication concentration (MBEC) adapted assay
Biofilm cell survival
The reduction in CFU for S. aureus and P. aeruginosa biofilm following treatment with ChloraSolv and Prontosan in the adapted MBEC assay is illustrated in Figures 1A–B. Following a 2-minute treatment with Prontosan, reductions in CFUs of 2.2 log10 and 1.4 log10 were observed for 48-hour biofilms of S. aureus (Figure 1A) and P. aeruginosa (Figure 1B), respectively. In contrast, after 2 minutes treatment with ChloraSolv, both S. aureus and P. aeruginosa biofilm was completely eradicated (>8 log10 reductions) to below the limit of detection which is significantly different to result obtained by Prontosan (p=0.011 and p=0.004 respectively). While further reductions in CFUs were observed at later time points (5 and 15 minutes) following treatment with Prontosan, the reductions were markedly less than observed for ChloraSolv.
Biofilm live-dead staining
Live-dead staining of S. aureus and P. aeruginosa biofilm before treatment showed cells to be entirely green, indicating that cells were all viable (Figures 2A and 3A). Following the treatment of S. aureus biofilm with ChloraSolv there was an increase in cell killing with exposure time (Figures 2B–E) After 30 seconds (Figure 2B) and 2 minutes of exposure to ChloraSolv (Figure 2C), some red (dead) cells and some yellow colour (a mixture and superimposition of green (live) and red (dead)), were observed. After 5 minutes (Figure 2D) and 15 minutes exposure (Figure 2E), the biofilm was stained mainly red, indicating extensive biofilm cell killing. Likewise, live-dead staining of P. aeruginosa biofilm treated with ChloraSolv showed an increase in bacterial cell killing with exposure time (Figures 3B–E). After 30 seconds (Figure 3B), the stained biofilm had changed from green to yellow, indicating a mixture of viable and non-viable cells. After 2, 5 and 15 minutes of exposure to ChloraSolv(Figures 3C–E), the biofilm was stained entirely red, indicating full cell killing. The findings of the live-dead staining for S. aureus and P. aeruginosa after ChloraSolv treatment were consistent with viable counts data from the MBEC assay (Figures 1A–B).
Gauze biofilm model
MRSA biofilm
MRSA biofilm CFUs for the no-treatment control, negative controls, and following debridement treatment test products are illustrated in Figure 4. The antimicrobial solutions soak, debridement pad/wipe and ChloraSolv negative controls all resulted in small but significant reductions in MRSA biofilm counts compared with the no-treatment biofilm controls (3.0×108 ± 1.9×108 CFU/gauze; p=0.023, p=0.008 and p<0.001, respectively), indicating that the physical aspect of these procedures resulted in modest reductions of MRSA in this biofilm model.
All four antimicrobial solution soaks, Prontosan, Octenilin, Veriforte and Granudacyn, resulted in statistically significant reductions in MRSA biofilm (up to approximately 1 log10 reductions) compared with their negative control (p<0.001, p=0.001, p<0.001 and p=0.008, respectively). There was no statistically significant reduction in MRSA biofilm counts following treatment with Debrisoft compared with its negative control (p=0.070). In contrast, the combination of Prontosan Pad with Prontosan Wound Irrigation Solution (3.7 log10) and UCS wipes (0.9 log10) resulted in a statistically significant reduction in MRSA biofilm counts compared with their negative controls (p=0.022 and p=0.035 respectively; Figure 4).
Following treatment with ChloraSolv, a dramatic reduction in MRSA biofilm was observed compared with any other treatment, with levels falling below the limit of detection (>6 log10, p=0.007 compared with the ChloraSolv negative control; Figure 4). Treatment with ChloraSolv significantly reduced the amount of MRSA biofilm compared with Debrisoft, Octenilin, Veriforte and Granudacyn (p=0.033; p=0.046, p=0.020 and p=0.009 respectively).
Multidrug-resistant P. aeruginosa biofilm
RPA biofilm CFUs for the no-treatment control, negative controls, and following debridement treatment test products are illustrated in Figure 5. The antimicrobial solution soak and ChloraSolv negative control resulted in statistically significant reductions in RPA biofilm counts per gauze circle compared with the no-treatment controls (3.1×108 ± 1.8×108 biofilm cells per gauze circle) (p=0.007 and p=0.005, respectively; Figure 5). This again indicates that the physical aspect of these procedures resulted in modest reductions of RPA in this biofilm model. However, the pad/wipe negative control did not result in significantly lower counts than the biofilm controls (p=0.486). This indicated that a saline rinse alone (i.e., the pad/wipe negative control) had minimal effect on RPA counts in this biofilm model.
All antimicrobial solution soaks, Prontosan, Octenilin, Veriforte and Granudacyn, resulted in statistically significant reductions of approximately 1 log10 in RPA biofilm compared with their negative controls (p=0.008, p=0.009, p=0.005 and p=0.005 respectively; Figure 5). The three debridement pads, Debrisoft, UCS wipes and Prontosan Pad with Prontosan Wound Irrigation Solution, resulted in statistically significant reductions of RPA biofilm compared with no-treatment biofilm control (p=0.002, p<0.001 and p<0.001, respectively), but not their negative controls, though the difference for Prontosan Pad with Prontosan Wound Irrigation Solution was 2.6 log10 (Figure 5).
In contrast to the reductions observed for the other test treatments, ChloraSolv treatment resulted in a larger and significant reduction in RPA biofilm compared with the ChloraSolv negative control (p=0.001) and combined biofilm controls (p<0.001; Figure 5): a reduction of >6 log10 was observed resulting in levels close to the limit of detection.
DISCUSSION
The antibiofilm activity of ChloraSolv in the standard MBEC biofilm model was demonstrated to be rapid with significant kill of biofilm occurring as early as 30 seconds, and complete eradication after 2 minutes. This was a more rapid and more extensive kill than observed for Prontosan, where substantial biofilm bacteria remained even after 15 minutes of treatment. When compared with a range of debridement methods including both antimicrobial solution soaks and physical removal with pads/wipes in a validated mature biofilm model, ChloraSolv demonstrated markedly greater reductions in biofilm cell counts of MRSA and RPA than observed for any other product as tested.
The slower kill rate of Prontosan was also previously observed by Krasowski et al (2021), where 24-hour S. aureus and P. aeruginosa biofilms grown on polystyrene plates were only eradicated after treatment for 24 hours. Rapid and effective reduction of biofilm and continued prevention of its re-formation is one of the cornerstones of Wound Hygiene (Murphy et al, 2020). Therefore, a debridement product that quickly and effectively reduces biofilm may contribute to more effective Wound Hygiene, resulting in improved wound outcomes, patient satisfaction, and more efficient clinical care with associated economic benefits.
Prontosan contains a betaine surfactant, which can reduce biofilm surface tension to allow the PHMB antiseptic to impart antibiofilm activity over time provided that sustained contact is ensured. Similarly, octenidine hydrochloride (in Octenilin) is a surfactant with antimicrobial properties. This contrasts with ChloraSolv’s rapid oxidative mode of action: ChloraSolv contains buffered 0.45% hypochlorite, which imparts antimicrobial activity due to oxidative and alkaline (high pH) properties (Estrela et al, 2002). Other oxidative wound cleansers/irrigation solutions include hypochlorous acid (e.g., Veriforte) and hypochlorous acid and sodium hypochlorite (e.g., Granudacyn). The four antimicrobial solution soaks tested in the validated gauze biofilm model gave modest reductions after 15 minutes (up to approximately 1 log10 compared with the controls). Elsewhere, similarly, modest decreases in biofilm were also previously reported in an in vivo porcine model of partial-thickness wounds, in which 24-hour biofilm was treated twice daily with antimicrobial solution soaks (including Prontosan, Octenilin and a hypochlorous acid and sodium hypochlorite cleanser). After 3 to 6 days (i.e., 6 to 12 treatments), the biofilm decreased, but only by approximately 1–2.5 log respectively against MRSA biofilm (Davis et al, 2017).
Alternative methods to antimicrobial solution soaks have emerged in recent years in the form of simple-to-use physical/mechanical debridement devices, such as pads and cloths. The gauze biofilm testing described herein also evaluated a monofilament pad (Debrisoft), a surfactant-impregnated wipe (UCS), and a microfiber pad that is used in conjunction with an antimicrobial irrigation solution (Prontosan Pad). As observed for the antimicrobial solution soaks, modest biofilm reduction was observed after use of the monofilament pad and surfactant-impregnated wipe. Previous ex vivo porcine model testing performed by Schultz et al (2018), using debridement tools including Debrisoft, showed an approximate 1.5 log10 reduction in P. aeruginosa biofilm. Those results are consistent with the findings from the present study where Debrisoft resulted in approximately 1 log10 reduction of P. aeruginosa biofilm. The slightly lower reduction in biofilm bacteria observed in the present study could be attributed to the higher starting inoculum compared with the previous study (approximately 1×108 CFU here compared with 1×106 CFU in Schultz et al, 2018).
It was observed that when a physical debridement product was used in conjunction with an antimicrobial solution, a further decrease in biofilm numbers was obtained. Although a notable decrease in biofilm numbers for both challenge microorganisms were observed with this combination, it was still notably less than the reduction in bacterial numbers that was observed following 2 x 2 minutes of ChloraSolv application (>6 log10 reductions). This notably enhanced antibiofilm activity may be attributed to the mode of action of ChloraSolv, with its oxidative activity against structural biofilm extracellular polymeric substances (polysaccharides, proteins, extracellular DNA), and cell wall and intracellular components of microbial cells (Zehnder et al, 2002).
Limitations
The main limitation of this study is that the testing performed was in vitro so cannot account for other physiological factors involved in wounds, such as the immune system, patient comorbidities and patient care protocols. As discussed above, the mode of application of antimicrobial solutions in other studies in the literature varies, which means comparisons between the data herein and data in the literature are difficult to make. Comparing physical debridement tools is also a challenge, as performing this by hand lacks consistency and introduces subjectivity. To address this, a previous study used mechanical equipment to replicate physical motion (Wilkinson et al, 2016). Interestingly, another study that compared the consistency of biofilm debridement by hand between the same operator, laboratory technician and a clinical expert, concluded that there was a similar amount of biofilm removed by all three users, suggesting that a consistent method of user debridement is sufficient when comparing products (Schultz et al, 2018). Finally, the minimum acceptable number of experimental replicates in microbiological testing is commonly recognised as 3 (US Pharmacopeia USP-NF-1227, 2021), due to the manpower required for these labour-intensive methods, but this means that the power of statistical tests performed on data are limited.
While it is not known how well our in vitro evaluations translate to clinical practice, there have been two clinical studies that evaluated the effect of ChloraSolv. The first was a prospective, multi-centre, randomised controlled study in patients with clinically infected diabetic foot ulcers (DFUs), where wound healing following ChloraSolv application for 12 weeks was compared with standard of care (SoC) (Bergqvist et al, 2016). Patients were followed for 24 weeks and included 17 patients in each arm. In the ChloraSolv arm, a statistically significant absolute reduction in DFU area relative to baseline was observed after only 2 weeks but was not observed until 8 weeks in the SoC arm. After 9 weeks, 7 patients had healed in the ChloraSolv arm, compared with only one in the SoC arm (p=0.039). The approximate relative decrease in wound area per week was 19.4% in the ChloraSolv arm and 11.7% in the SoC arm.
The second study was a prospective, multi-centre, single-arm clinical study, to evaluate the debriding effect of ChloraSolv on 57 lower leg ulcers, with at least 50% devitalised tissue coverage, after 6 weekly treatments during a 5-week period with a 12-week follow up (Eliasson et al, 2021). After ChloraSolv treatment, devitalised tissue coverage was reduced by 72.7% at week 5 and by 84.4% at week 12. Pain levels were reported as good or very good by 90% of patients, while 70% of the investigators or nurses rated ChloraSolv easier to use than previous debridement methods.
CONCLUSIONS
In increasingly complex in vitro biofilm tests, ChloraSolv showed notably greater antibiofilm activity compared with other debridement techniques/products. Although this study only examined the in vitro antibiofilm activity of ChloraSolv, in conjunction with recent clinical studies which focused on easier debridement of devitalised tissue, it highlights the promise for this new wound debridement gel which combines tissue softening and antibiofilm activity. Effective wound debridement that is quick and easy to perform, without the requirement for specialist training, is critical to ensure Wound Hygiene is routinely available to patients with hard-to-heal wounds.