In the UK approximately 11 million surgical procedures or interventions are performed annually (DH, 2009; ONS, 2010). Most result in a break in the natural protective barrier provided by the skin, increasing the risk of contamination from exogenous or endogenous bacteria.Postoperative wound sepsis carries high morbidity and additional costs. However, most postoperative complications are preventable and their incidence can be reduced by taking appropriate measures in the pre, intra- and postoperative phases of care to reduce the risk of infection. This made easy reviews the strategies that can minimise the risk of postoperative complications and offers recommendations for best practice, based on the latest NICE guidance (2008) and expert opinion, on effective postoperative incision management.
Understanding the risk of postoperative complications
Most surgical wounds are categorised as acute wounds, healing without complication in an expected timeframe. However, like all wounds, healing may be affected by intrinsic and extrinsic factors that may result in postoperative wound complications, such as surgical site infections (SSIs), which delay healing (Baxter, 2003).
It has been highlighted that there may be a misperception among some surgeons that these complications are rare (Clarke et al, 2009). In the UK, infection rates vary greatly between different types of surgery, ranging from 0.6% to 0.8% after knee and hip prosthetic surgery, to 10.1% and above for surgery involving the bowel or limb amputation (HPA, 2011). The difference in these figures may relate to whether the surgical procedure was clean, clean-contaminated or contaminated.
However, these figures are considered to be an under-estimation as they include inpatient and readmission data only, and there is limited provision for reporting and follow-up in the community. The trend towards shorter hospital stays means that infection rate figures often do not reflect wound breakdown and infections that occur once the patient has been discharged. For example, Reilly et al (2006) found that for breast surgery, Caesarean section, hip replacement, and abdominal hysterectomy, the rate of SSI when post-discharge surveillance (PDS) was performed was significantly higher than that when it was not.

Surgical site infection
Surgical site infection (SSI) is the most common postoperative incisional complication (others include postoperative blistering and wound dehiscence, which may often be related to SSI) and comprises approximately 20% of all healthcare associated infections (HCAIs). At least 5% of patients develop an SSI after a surgical procedure (NICE, 2008).
An SSI can range from a spontaneously limited wound discharge, recognised usually within 7-10 days of an operation, to a life-threatening postoperative complication, such as abdominal wound dehiscence or a sternal infection with mediastinitis and dehiscence after open heart surgery.
An SSI can have a considerable impact on a patient’s quality of life, carry a higher risk of morbidity and mortality, and lead to a prolonged hospital stay (Coello et al, 2005) or rehospitalisation with greater use of healthcare resources and higher costs. Based on an SSI rate of 5%, NICE (2008) estimated each episode to cost £3500, and the overall cost to the NHS of managing SSIs to be around £700 million per year.
Principles of managing postoperative incisions
A multidisciplinary approach to postoperative care involving the surgical team is required to improve the overall management of surgical wounds.
To support clinicians, a strategy for the prevention of HCAIs, including SSIs, and specific guidelines have been issued to prevent and manage complications (Pratt et al, 2007; NICE, 2008). They highlight the importance of a thorough and structured approach to pre-, intra- (Table 1) and postoperative care.
This has led to the development of a High Impact Intervention (HII) care bundle, which is based on the 2008 NICE guidelines and expert advice. This comprises three clinical actions which, if all elements are performed every time and for every patient, will reduce the risk of infection. However, the risk of infection increases when one or more actions of a care bundle are excluded or not performed (DH, 2011). Regular auditing of the care bundle actions and review of clinical practices are aimed at improving the quality of care.

Preoperative phase
Patients at higher risk of postoperative incisional complications may be identified using a comprehensive preoperative assessment. Factors increasing a patient’s risk of wound healing problems, such as wound dehiscence or blistering, include poor nutritional status, obesity, smoking/living with a smoker, and belonging to particular patient groups. These include those with diabetes, rheumatoid arthritis and patients taking steroids or immunosuppressants.
Intraoperative phase
Operating staff are required to use an aseptic technique during surgical procedures and to prepare the skin at the surgical site immediately before incision using an antiseptic preparation. Surgical incisions anticipated to heal by primary intention should be covered by a film membrane, with or without a central absorbent pad (NICE, 2008). This should be left in place for 3-5 days providing no adverse events occur (eg wound pain, pyrexia or wound discharge).
Postoperative phase
After the initial postoperative phase (3-5 days) recommendations include:

- Use an aseptic, non-touch technique for changing and removing dressings.
- Keep the frequency of dressing changes to a minimum to avoid disrupting healing tissue.
- Use sterile saline for wound cleansing up to 48 hours after surgery.
- Use tap water for wound cleansing after 48 hours if the wound has separated or has been surgically opened to drain pus. Antiseptic agents are considered unnecessary for general wound cleansing but may be of value when irrigating an infected cavity wound.
- Where periwound skin maceration occurs or is considered to be a risk (eg if an enteral fistula is present or if there are excessive exudate levels) consider skin barrier products.
- Use an interactive dressing (ie one that promotes the wound healing process through the creation and maintenance of a local, warm, moist environment underneath the chosen dressing) for surgical wounds that are healing by secondary intention (NICE, 2008). The dressing should be left in place for as long as indicated. A continual assessment process ensures dressing changes are kept to a minimum.
- Advise patients that they can shower safely 48 hours after surgery.
- Do not use topical antimicrobial agents for surgical wounds that are healing by primary intention.
- Refer the patient to wound care specialists if required for advice on appropriate dressings and care
- Educate patients and carers and other healthcare professionals on optimal wound care, how to identify a wound that is failing to heal and who to contact if they are concerned about a possible SSI (NICE, 2008).
A postoperative dressing should be removed earlier than the recommended 48 hours if there are clear signs of complications, eg signs of excessive inflammation which may suggest infection, specific wound pain or pressure reported by the patient that is difficult to control with analgesia, evidence of wound separation (partial or full thickness dehiscence), excessive exudate, strikethrough or leakage, or evidence of periwound skin stripping or blisters (Bhattacharyya et al, 2005; Cosker at al 2005).
If an SSI is suspected (ie cellulitis or a collection of pus with systemic complications such as sepsis), antibiotics may need to be considered. Intervention and release of pus must be a priority. Antibiotic choice should be based on the most likely causative organisms and patient allergy status, with consideration given to local antibiotic resistance patterns and, when possible, the results of available microbiological culture and sensitivity tests from the patient as a whole.

Dressing selection for wounds healing by primary intention
Dressing choice can significantly affect the outcome of postoperative wound healing and dressings should be chosen to optimise healing and minimise complications (eg select a dressing associated with a lower incidence of blistering) (Cosker et al, 2005).
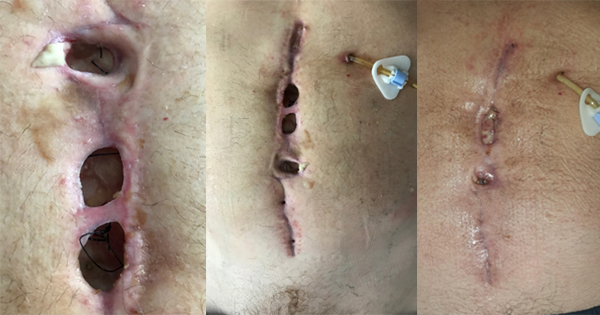
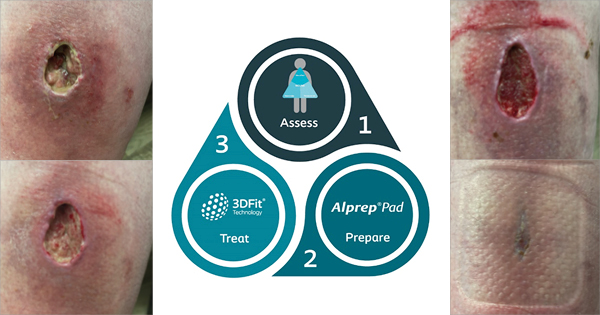
Ideally dressings should maintain a moist wound environment conducive to optimal healing, while avoiding maceration or blistering of the surrounding skin (Figure 1). The choice of dressing depends on wound type, position and size/depth. Other points to consider are the range of dressing sizes available, conformability and acceptability to the patient. When placing a dressing careful consideration should be given to dressing orientation and tension and how patient movement postoperatively may affect this. This can be a significant problem when dressing wounds over joints where movement can result in skin damage and blistering due to shear (Leal and Kirby, 2008) (Figure 2).
Cosker et al (2005) set out the combination of features and properties of an ideal postoperative wound dressing that aims to provide good wound care and reduce the risk of postoperative complications.
Implementing NICE guidance on postoperative dressing selection
Where possible postoperative dressing choice should be aligned with NICE 2008 guidance or, where applicable, evidence-based guidelines. Low-adherent postoperative dressings or vapour-permeable polyurethane ?lm dressings are usually used for uncomplicated surgical wounds with or without an incorporated, absorptive, central ‘island’ pad. However, dressing practice may differ and include the use of various types of wound dressing or coverings, such as non-woven dressings, or simple gauze held in place by tape. Increased rates of blistering are associated with tape methods (Bhattacharyya et al, 2005) and a significantly lower (p<0.001) incidence of blistering has been described when using a vapour-permeable polyurethane film dressing (Cosker at al, 2005). Vapour-permeable film dressings offer a number of advantages over non-woven dressings (Roberts et al, 2011) in that they:
- Provide a barrier to extrinsic contamination
- Allow postoperative inspection of the periwound area (or inspection of the wound itself) without removal of the dressing in the first 24-48 hours
- Allow easy removal as a result of low adhesion to the wound
- Maintain a moist wound environment
- Enable showering (ie are waterproof)
- Can be left in place for up to 7 days.
- Are conformable to body contours and tend to be more stretchy, allowing for postoperative movement/wearer comfort with reduced incidence of blistering.
Evidence base for following NICE guidance
A multicentre clinical study evaluated the performance of a vapour-permeable transparent film post-surgical dressing (OPSITETM Post-Op Visible, Smith & Nephew) in a typical clinical setting (O’Brien et al, 2010). Sixty-four patients who underwent clean surgery were treated with the film dressing. Duration of dressing wear, visibility through the dressing and ability to handle exudate were assessed and the product was rated in comparison to those normally used. Mean wear time was 4.5 days. Exudate management was rated very good or good at 96% of assessments. Visibility of the incision site was rated as very good or good at 72%, and as acceptable at 24% of assessments. Patient comfort was rated as very comfortable (63%) or comfortable (37%) at all assessments. Dressings were generally rated as satisfactory or exceeding expectations.
In a survey of postoperative dressing practice pre- and post-implementation of the 2008 NICE guidelines, Roberts et al (2011) reviewed 78 incisions before the change in practice, where the wounds were dressed with non-woven dressings, and 104 incisions where the wounds were dressed with vapour-permeable film dressings after the change in practice. They compared nine criteria, and in eight of these, film dressings were rated as being superior. The authors concluded that the vapour-permeable film dressing had a considerable advantage over non-woven dressings, which do not have a ‘see-through’ central absorbent pad. They suggested that as clinicians were able to clearly visualise the incision site while the dressing was in place, they were able to inspect the surgical site for evidence of complications. This offers a distinct advantage in detecting signs of wound complications and infection at an early stage, which can often be difficult because the wound is usually obscured by the dressing (Tustanowski, 2009).
Although Roberts et al (2011) reported an increase in the cost of film dressing per patient by £2.53 compared with non-woven dressings, they found that fewer dressing changes were needed when applying the film dressing, which had a positive impact on staff time and indirectly reduced overall costs.
Managing complex surgical wounds
Most postoperative wounds will usually heal within 7-14 days depending on the type of surgery. Despite best practice, some surgical wounds fail to heal primarily or are deliberately left open to heal by secondary intention. Several tools exist to optimise healing by secondary intention. Wound bed preparation using the TIME concept, as first described by Shultz et al (2003) and its subsequent revisions (Dowsett and Ayello 2004; Leaper at al, 2012), is a practical tool for identifying barriers to healing and implementing a treatment plan to promote wound healing.
To improve the onward management of complex surgical wounds NICE (2008) suggests the onward referral to a tissue viability nurse (or another healthcare professional with tissue viability expertise) for advice on appropriate dressings for surgical wounds that either dehisce postoperatively or are electively left open to heal by secondary intention (eg pilonidal sinus). Negative pressure wound therapy may also be considered for more complex wounds such as abdominal wound dehiscence (WUWHS, 2008).
Summary
With the drive for ever shorter hospital stays and community care, staff education and reporting systems need to be in place so that clinicians are equipped to deal with postoperative complications and data reported so that real world practice can be monitored and improved. Choice of dressing can significantly affect the outcome of healing in patients with postoperative incisions. A postoperative wound dressing should not be arbitrary, nor based solely on the initial cost of the dressing (Cosker et al, 2005). Effective wound management will expedite and optimise healing, and reduce rates of complications that adversely affect patients’ quality of life and healthcare costs.
References
- Baxter H (2003) Management of surgical wounds. Nursing Times 99(13): 66-68
- Bhattacharyya M, Bradley H, Holder S, Gerber B (2005) A prospective clinical audit of patient dressing choice for post-op arthroscopy wounds. Wounds UK 1(1): 30-4
- Bratzler D, Houck PM (2004) Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 38(12): 1706-15
- Bratzler DW, Hunt DR (2006) The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis 43(3): 322-330
- Clarke JV, Deakin AH, Dillon JM, et al (2009) A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care 18(1): 5-8, 10-1
- Cosker T, Elsayed S, Gupta S, et al (2005) Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care 14(1): 27-29
- Coello R, Charlett A, Wilson J, et al (2005) Adverse impact of surgical site infections in English hospitals. J Hosp Infect 60(2): 93-103
- Dowsett C, Ayello E (2004) TIME principles of chronic wound bed preparation and treatment. Br J Nurs 13(15): S16-S23
- Department of Health (2009) Hospital episode statistics. The Health and Social Care Information Centre, London. Available at: http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937
- Department of Health (2011) High Impact Intervention Number 4. Care bundle to prevent surgical site nfection. London: DH. Available from: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_078123.pdf
- Health Protection Agency (2011) Surveillance of surgical site infections in NHS Hospitals in England 2010/2011. Available from http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1317131662062
- Leal A, Kirby P (2008) Blister formation on primary wound closure sites: a comparison of two dressings. Wounds UK 4(2): 31-37
- Leaper DJ, Schultz G, Carville K, et al (2012) Extending the TIME concept: what have we learned in the past 10 years? Int Wound J In press
- O’Brien G, Buckley K, Vanwalleghem G, et al (2010) A multi-centre, prospective, clinical in-market evaluation to assess the performance of Opsite Post-Op Visible dressings. Int Wound J 7(5): 329-37
- Office for National Statistics (2010) Population estimates for UK, England and Wales, Scotland and Northern Ireland. HMSO, London. Available from: http://www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk–england-and-wales–scotland-and-northern-ireland/index.html
- National Institute for Health and Clinical Excellence (2008) Quick reference guide: surgical site infection. London: NICE. http://www.nice.org.uk/nicemedia/live/11743/42381/42381.pdf
- Pratt RJ, Pellowe CM, Wilson JA, et al (2007) epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hospital Infect 65: S19-S22
- Reilly J, Allardice GM, Bruce J, et al (2006) Procedure-specific surgical site infection rates and postdischarge surveillance in Scotland. Infect Control Hosp Epidemiol 27(12): 1318-23
- Roberts N, Sorrell J, Bielby A, Searle A (2011) A survey of postoperative wound dressing practice before and after implementing national guidelines. Wounds UK 7(4): 12-21
- Schultz GS, Sibbald RG, Falanga V, et al (2003) Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 11(1): S1-28
- Tustanowski J (2009) Effect of dressing choice on outcomes after hip and knee arthroplasty: a literature review. J Wound Care 18(11): 449-50, 252, 454
- WUWHS (2008) Principles of best practice: vacuum assisted closure. Recommendations for practice. MEP Ltd: London. Available from www.woundsinternational.com
AUTHOR DETAILS
Milne J[1], Vowden P[2], Fumarola S[3], Leaper D[4]
1. Tissue Viability Nurse Specialist, South Tyneside NHS Foundation Trust
2. Vascular Surgeon, Bradford Teaching Hospitals
3. Senior Clinical Nurse Specialist, University Hospital of North Staffordshire
4. Visiting Professor, Cardiff UniversitySupported by Smith & Nephew