Phlegmasia Cerulea Dolens (PCD; literal translation: ‘painful blue oedema’) is a rare but limb- and life-threatening complication of Deep Venous thrombosis (DVT) of the extremities (Lipe and Cuthbert, 2017). Clinical presentation is an acute and severe swelling of the extremities, predominantly the left lower limb along with ischaemic pain (arterial spasms) and cyanosis (Fong et al, 2018). The mortality rate is 25-40% and major amputation rate is 20-50%; due to the rarity of the condition, the incidence is unknown (Chaochankit and Akaraborworn, 2018). A descriptive systematic analysis of the literature by Chinsakachi et al (2011) revealed hyper-coagulable state as a significant risk factor for PCD with malignancy being the most common risk factor. Notably, the left limb was affected more (in 45% of cases) and the popliteal vein in 6% of cases (total patients = 62).
Differential diagnoses include lymphatic obstruction, acute cellulitis and acute arterial occlusion (Chaochankit and Akaraborworn, 2018). Pathogenesis comprises of near or total venous occlusion by a clot of the major deep veins of the extremities. The resulting increase in hydrostatic pressure within the limb extends to the collateral veins, causing severe venous congestion. Cyanosis is a consequence of an accumulation of carboxyhaemoglobin in the veins supplying the dermis (Lipe and Cuthbert, 2017). Gangrene may ensue, secondary to inadequate tissue perfusion.
Critically, life- and limb-saving interventions are the immediate restoration of the venous circulation by relieving the severe pressure on the limbs. Medical intervention includes thrombolysis, thrombectomy or fasciotomy. Current recommended invasive therapies are: catheter-directed thrombolysis, percutaneous mechanical thrombectomy and pharmaco-mechanical catheter-directed thrombolysis (Elsaid et al, 2019). The goal of medical treatment is to preserve the patency of the venous collateral circulation, preserve venous valve function and prevent risks for recurrence (Chaochankit and Akaraborworn, 2018).
Case presentation
A forty-two-year-old male was admitted to hospital with idiopathic thrombocytopaenia (platelets <10). Regular warfarin treatment was held until platelet count reached >50. He subsequently developed severe pain, progressive and extensive swelling, oedema and cyanosis to the left lower limb. Due to pain, he was unable to bear weight. Pedal pulse was present but diminished. Motor and sensory functions remained intact.
A past medical history was significant: liver transplants x 2, previous deep vein thromboses (DVTs), Sjogren’s Syndrome, pulmonary embolism and idiopathic thrombocytopaenia. A known allergy was penicillin and sensitivity to morphine. An emergency fasciotomy procedure was undertaken by the surgical team on the left lower limb to relieve excess hydrostatic pressure on the venous circulation due to compartment syndrome. Unfortunately, the patient developed a severe reaction to the general anaesthetic during surgery, thereby ruling out further surgical intervention. Therefore, skin grafting to close the open wounds was not possible. Post-operatively, therapeutic anticoagulation therapy was initiated along with limb elevation and bed rest (Hinojosaet al, 2016).
Blood cultures revealed gram negative growth while a wound swab showed pseudomonas species. Antibiotic treatment was given: Daptomycin and Meropenem. Multiple radiology investigations ruled out necrotising fasciitis of the limb. An ultrasound doppler venogram revealed a popliteal DVT. The left common femoral vein and left superficial femoral vein were patent and compressible. An x-ray of the left tibia and fibula showed no subcutaneous emphysema. Diffuse subcutaneous thickening and oedema was revealed by magnetic resonance imaging, indicating cellulitis.
Wound assessment
Postoperatively, the patient was referred to the Tissue Viability Nurse (TVN) Specialist Service for advice and management of the open fasciotomy wounds. Using the Triangle of Wound Assessment (Dowsett et al, 2015) as a conceptual framework and guide, the TVN carried out a full and holistic assessment of the patient and the wound. A care pathway, discussed herein, comprised of mixed wound treatment modalities to achieve complete healing as an outcome. The intention was to treat and heal the wounds and regain functionality of the limb with the patient as an active participant in the treatment process.
A focused clinical assessment revealed three open fasciotomy wounds present to the left lower limb. Motor and sensory functions were assessed as intact. There was extensive oedema from mid-thigh to forefoot. Capillary refill was present to the toes (<5 seconds).
Recorded wound dimensions were as follows:
- a distal wound to the dorsum of the left forefoot: 8cm long x 4.5cm wide
- a wound to the middle of the lateral aspect of the limb: 8.2cm long x 5cm wide
- a wound to the proximal aspect of the limb: 12cm long x 4.5cm wide.
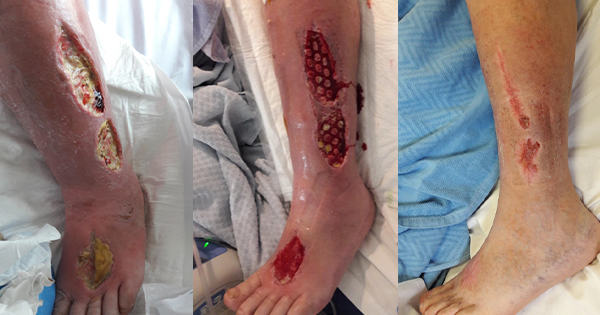
A layer of deep, firmly adherent, dark yellow slough (100%) was present on all wound beds, as illustrated in Figure 1. This was accompanied by a continuous large volume exudate of serosanguinous fluid. Wound infection was clinically suspected by wound margin erythema and oedema from the mid-thigh to foot, along with pocketing in the wound bed. Wound edges were irregularly shaped and macerated secondary to copious wound exudate levels.
Following the patient and wound assessment, a comprehensive wound management plan was developed and discussed in collaboration with the patient, the surgical team and the ward staff. The main goal was to maintain function of the lower limb, while the objectives were to remove non-viable tissue, manage wound exudate and reduce bacterial burden and oedema.
It was anticipated that management of the wounds and the healing outcomes would take a considerable length of time. The patient and his family were counselled regarding the wound management interventions and involved in the care planning process. Appropriate analgesia was administered ahead of wound management interventions.
Wound management interventions
Exudate management was initially achieved using superabsorbent products and a three-layer, outer-stocking support system. The copious volume of exudate necessitated at least twice-daily changes for 5 days. After 1 week, exudate levels reduced and larvae therapy was applied on all wounds for 3 weeks to debride non-viable tissue (Rafter, 2013) [Figure 2]. Coagulation results, measured as International Normalised Ratio (INR), were noted and wounds were carefully monitored for excess bleeding.
At week four, Negative Pressure Wound Therapy with instillation (NPWTi) with reticulated open-foam dressing was applied under light compression therapy, bandaging for a total of 7 days. The NPWTi facilitated wound bed contraction, cleansing of debris and rapid growth of granulation tissue. The defect began to fill up to skin level with granulation tissue and comedone growth [Figure 3].
At week five, a standard NPWT without instillation was applied under light compression bandaging once granulation tissue was evident. A protease modulating dressing (containing collagen and silver) was applied to all wound beds under the NPWT to promote faster growth of epithelial tissue.
Once clinically stable, the patient was discharged home and followed up in the Outpatients Wound Management clinic by the TVN.
Outcomes
A light compression therapy system was applied to promote healing in conjunction with NPWT.
By week eight, the wound to the dorsum of the left forefoot closed over successfully. At this stage, the patient’s pain control had improved significantly. Following 12 weeks of treatment, there was an average of a 61% reduction in all wound sizes [Figure 4]. Full compression bandaging was applied to promote venous return and aid closure of the remaining wounds.
Careful discharge planning factored in patient’s acceptance of compression bandaging and planned regular attendances at the Dressing Clinic for ongoing reviews. After five months, all wounds had completely epithelialised. Lymphoedema was diagnosed in the limb and was managed by bespoke compression wraps in collaboration with the Lymphoedema Service. In this case, complete wound healing as an outcome was achieved while the limb and its full function were preserved. The patient returned to full activities of daily living.
Discussion
Medical management of PCD is challenged by the limited knowledge around the condition. Moreover, a lack of clinical guidelines and no gold standard for treatment can lead to inconsistent treatment/management (Chinsakchai et al, 2011).
To manage, along with patient discomfort, large-volume wound exudate was a challenge immediately after surgery for the nursing staff. Lipe and Cuthbert (2017) report that, secondary to PCD, volumes of 6-10 litres of fluid may develop within the limb. Additionally, excess volumes of chronic wound exudate damage the growth factors necessary for wound healing by altering the biochemical composition of the wound fluid and wound bed, thus preventing angiogenesis (Schultz et al, 2003). Skin grafting to close the wounds was not possible due to severe reactions to a previous general anaesthetic; therefore, wound debridement was achieved using combined larvae therapy and NPWTi. Larvae therapy is a recognised first-line treatment in chronic wounds of the lower limb (McIntosh et al, 2019)(Rafter, 2013). Ammonia, secreted by larvae, increases wound pH, thereby exerting antibacterial activity, notably against methicillin-resistant Staphylococcus aureus (King, 2020). Additionally, proteases secreted by the larvae break and liquify necrotic tissue. In this case study, the larval therapy was part of the initial wound bed preparation for the healing trajectory. Interestingly, a recent literature review yielded few results on the promotion/usage by clinicians of larvae therapy for wound management (King, 2020). Both semantics and clinicians’ perceptions may influence clinicians’ decision of not using this effective bio-surgical therapy as part of wound bed preparation.
Despite routine use in clinical practice to facilitate wound healing/closure, there is a lack of rigorous randomised controlled trials (RCTs) on the clinical effectiveness of NPWT. This is reflected in a systematic review of NPWT for open surgical wounds healing by secondary intention (Dumville et al, 2015). The review yielded low-quality RCTs where the outcome was healing rates. Despite the apparent lack of empirical evidence for the effectiveness of NPWT for wound healing, the system is widely used in clinical practice where anecdotal evidence yields benefits such as wound closure, exudate management, removal of infectious material and a reduction in nursing time and resource useage (e.g. dressing products) (Kieser et al, 2011). Specifically, the foam used with the NPWTi system is denser with increased tensile strength and greater fluid distribution along the wound bed (Wolvos, 2015).
Conclusion
PCD is a rare but, potentially life- and limb- threatening condition that can rapidly progress to compartment syndrome and gangrene of the affected limb. It is a rare complication of DVT in patients with hypercoagulable states. Moreover, the current lack of clinical guidelines makes management of this condition challenging.
Under the leadership of the TVN service, planned and co-ordinated wound therapies were implemented to clean the wound beds, manage large-volume exudate and close the wounds. The patient was able to return to full mobility and activities of living.