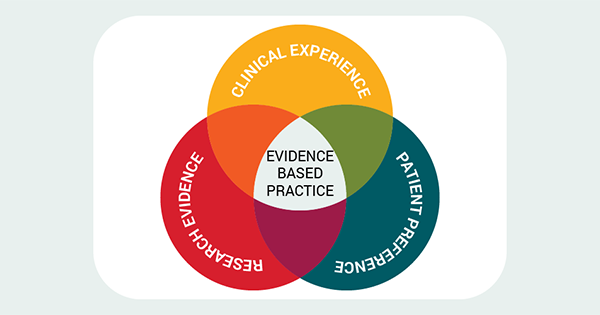
In the last paper in this series we continued to consider how one might assess the quality of quantitative research with reference to a randomised controlled trial (RCT; Ellis, 2022a). We considered the features of sampling and randomisation that researchers should explain and which tell the reader a lot about the applicability, generalisability, of the study to the sorts of people they care for. We saw how, as part of this requirement, the characteristics of the study sample need to be explained so that the healthcare professional can make this assessment.