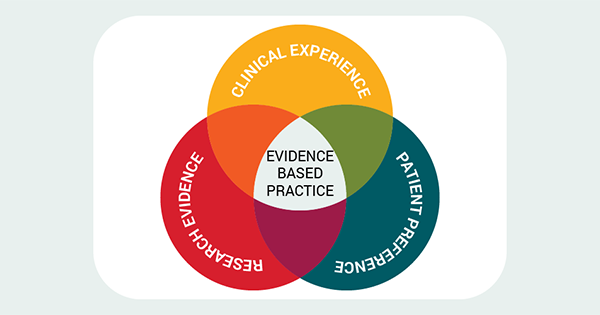
The treatment of individuals who suffer from venous leg ulceration (VLU) account for a sizeable portion of the total number of wounds that are seen in the United Kingdom (UK) (Guest et al, 2015; Guest, 2020). Despite empirical evidence and national treatment recommendations, there are considerable variations in clinical practice and services across the UK. These differences result in delays in diagnosis, ineffective or inappropriate treatment plans, increased costs for health services, a greater burden on clinical resources, and an extended negative impact on patient quality of life due to extended healing times.
This paper defines evidence-based practice, presents a summary of the evidence base for effective leg ulceration management, and focuses on examining the evidence for strong compression therapy and venous ablation, which are included in the National Wound Care Strategy Programme (NWCSP, 2020). Additionally, it explores the recently renewed recommendation from NICE (NICE, 2023) regarding the potential benefits of using the Urgostart treatment range as a first-line dressing initiative. Finally, it will highlight how the use of clinical pathways can ensure patients receive evidence-based care consistently.
The overall burden VLU
The number of patients living with lower-limb ulceration is increasing year on year (Guest, 2020), with the number of diagnosed venous leg ulcers being 560 000 in 1.1% of all adults over 18 years of age. The overall expansion of prevalence has been linked to substantial increases in resources, overall costs increased by 48% within a 5 year time frame (Guest et al., 2020). The majority of these patients have been diagnosed with venous ulceration, but potentially more worryingly around 36% of patients with lower limb wounds did not have a recorded diagnosis. Potentially, this lack of diagnosis implies that a large proportion of patients could experience delayed wound healing due to the absence of effective treatment such as compression therapy. Additionally, the impact on the individual patient cannot be overlooked; experiencing a VLU is known to be connected with a detrimental impact on bio-psycho-social -spiritual and socioeconomic dimensions of quality of life (Joaquim et al, 2018).
Variation in care
Across the UK, there is considerable variation in care for patients with lower-limb ulceration; there is an underuse of evidence based practices and an overuse of ineffective practices (Guest et al, 2015; Murray and Norrie, 2020). These variations impact directly on patient outcomes, increasing care costs and extending healing times (NWCSP, 2023). However, this unwarranted variation offers major opportunities to improve healing rates, reduce recurrence rates, reduce individual suffering, reduce spending on inappropriate and ineffective treatments, and the amount of clinical time spent on care (NWCSP, 2023). Healing rates of patients with VLU within a community setting have been reported to be as little as 37% at 12 months (Guest, 2020). Time to healing has been reported to be shorter when care is aligned with the evidence base (Gohel et al, 2018) where healing rates were reported to be over 85% at 24 weeks (Gohel et al, 2018). Although Gohel’s paper focusses on one specific intervention that of a comparison of early endovenous ablation vs. deferred endovenous ablation of superficial venous reflux it clearly demonstrates the importance of using evidence-based interventions. There is an urgent need to improve access to evidence based practice, providing opportunities to improve healing rates, reduce costs to the NHS and reduce patient suffering.
What is Evidence-based practice
Evidence-based practice (EBP) was defined by Sackett et al (1996) as the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients and was updated in the Sicily statement on evidence-based practice (Dawes et al, 2005). They extended the definition as decisions about health care, which are based on the best available, current, valid and relevant evidence, and that decisions should be made by those receiving care, informed by the tacit and explicit knowledge of those providing care, within the context of available resources. Sometimes there is an insufficient research base for clinicians to access meaning healthcare decisions are based on non-research evidence sources and scientific principles (Titler et al, 2001) including guidelines, expert opinion, best practice and consensus documents.
A vital aspect of EBP is the clinician’s ability to be able to critically appraise and analyse both research and non research evidence to determine its validity and applicability to clinical practice. Connor et al (2023) in their scoping review exploring if EBP can improve patient outcomes concluded EBP can improve patient outcomes and delivers a return on investment for healthcare systems. However, they do recommend clinicians, academics and publishers take a professional responsibility to highlight and explore confusion surrounding EBP and make clear the differences between quality improvement, implementation science, EBP, and research.
Venous Ulceration
A leg ulcer is defined as a break in the skin below the knee but above the malleolus that has not healed within 2 weeks (NWCSP, 2023). A venous leg ulcer occurs in the presence of venous disease and is the most common type of leg ulcer, accounting for 60–80% of cases and typically occurs in the gaiter area of the leg (from the ankle to mid-calf) (NICE, 2024). Venous disease, or venous insufficiency is often the result of the valves within the veins not working effectively and instead allowing backflow, resulting in abnormal venous pressure (venous hypertension). Matrix Metalloproteinases (MMPs) are part of normal wound healing and play a key role in the inflammatory phase of healing for cell proliferation (Krishnaswamy et al., 2017). However, prolonged elevated levels of MMPs are linked to slow healing (Raffetto et al., 2021). It is known that MMP’s are already at higher levels in wounds of patients with venous insufficiency, leading to a persistent inflammatory state (Raffetto, 2016). Vein function can be affected by:
- Trauma
- Venous obstruction
- Inactivation of calf/foot muscle pump failure
- Varicose veins that cause failure of the venous valves
- Venous thrombosis.
Venous pressure has a significant physiological influence, and this chronic condition results in the release of several chemical inflammatory mediators. This affects fluid balance in the capillaries and surrounding tissue, pushing fluid, proteins, and blood products out of the vein and into the surrounding tissue (Ortega et al., 2021). The long-term effects of this condition include oedema due to localised tissue inflammation, skin pigment changes (haemosiderin staining), tissue fibrosis (lipodermatosclerosis), and the potential for cellular hypoxia and ulcer formation.
Summary of evidence: Compression therapy
Compression therapy generates external pressure on superficial veins and tissues, thereby assisting venous return to reduce peripheral oedema and promote wound healing on the lower limb (Nair, 2014). This is a result of reduced vessel diameter, which is achieved through compression therapy, which increases the venous velocity (Partsch and Mortimer, 2015). However, it is widely recognised that compression therapy goes beyond simply reducing venous hypertension, and oedema playing an important role in tissue remodelling (Conde Montero et al., 2020).
Conde Montero state the main effects of compression therapy are:
- Reduction in oedema
- Tissue remodelling
- Reduced filtration of fluid from vessels to tissue
- Increased lymphatic drainage
- Increased levels of anti-inflammatory mediators
- Reduction in inflammatory molecules (Conde Montero et al., 2020, Altintas et al., 2011, Beidler et al., 2009, Beidler et al., 2008)
Strong compression therapy delivering at least 40 mmHg of pressure at the ankle is the recommended first-line treatment for venous leg ulcers (O’Meara et al., 2012). Strong compression therapy is known to decrease the time to healing and reduce the risk of recurrence (Ashby et al., 2014, NWCSP, 2023, O’Meara et al., 2012) . There is a statistically significant difference in healing rates when compression is used compared with no compression (Patton et al., 2023, Shi et al., 2021).
Summary of evidence: Venous ablation
Traditional varicose vein treatments involved ligation and stripping of the superficial veins, but in recent decades, minimally invasive treatments like Radiofrequency Ablation (RFA), endovenous laser ablation (EVLA), and foam sclerotherapy have emerged. These innovative methods involve no vein removal or general anaesthesia (Paravastu et al., 2016), instead they ablate (destroy) the vein using heat or chemicals damaging the vein wall to create fibrotic occlusion and only require local anaesthesia. It has been demonstrated that treating superficial venous reflux in addition to compression therapy speeds up the healing process for VLUs and lowers the likelihood of recurrent ulcers (Gohel et al., 2018, Barwell et al., 2004). Gohel et al. (2018) conducted a significant randomised control trial (RCT) known as the EVRA trial to examine the effects of early venous ablations in individuals with venous ulcers. In this National Institute of Health and Research Health Technology (NIHR)-funded study, 450 patients were randomly assigned to either compression therapy and early endovenous ablation of their superficial venous reflux within two weeks of randomization (early intervention group) or compression therapy alone with consideration of endovenous ablation once the ulcer had healed or if the ulcer was still active at six months after randomization (deferred intervention group). The study’s primary endpoint was time to healing, with secondary outcomes including the rate of ulcer healing at 24 weeks, the rate of recurrence, the amount of time without an ulcer, and patient-reported health-related quality of life. The study found that the median time to healing decreased from 82 days to 56 days in the early intervention arm compared to the deferred intervention group (p=0.001) and that the amount of time without an ulcer improved. Additionally, venous intervention has been shown to decrease the rate of ulcer recurrence from 28% at 1 year to 12% (p=0.0001) (Barwell et al., 2004). Venous intervention is likely to be cost effective in patients with VLU at 1 year (Epstein et al., 2019). Wound dressings are used to manage VLU and NICE have recommended UrgoStart as a cost effective treatment underpinned by research and evidence.
Summary of evidence: Urgostart
UrgoStart Plus treatment range contains Technology Lipido-Colloid–Nano-Oligo Saccharide Factor (TLC-NOSF), which reduces matrix metalloproteinase (MMPs) and promotes angiogenesis through migration and proliferation of endothelial cells (Murray and Norrie, 2020, Edmonds et al., 2018, Maunoury et al., 2021). Urgostart Plus treatment range is an interactive dressing that has been shown to statistically improve time to complete wound closure when compared to standard wound dressings (Shanahan, 2013). Evidence has shown wound area reduction in the first 8 weeks, with venous leg ulcers typically achieving full wound closure within 18 to 24 weeks (Meaume et al., 2017), resulting in associated savings of £541 per patient per year. UrgoStart has been recommended by the National Institute for Health and Care Excellence (NICE) as a cost saving option to treat patients with diabetic foot ulcers and venous leg ulcers due to its proven efficacy in improving healing outcomes (NICE, 2023).
NICE guidelines should be viewed as an independent and robust review of the most up to date evidence that supports patient care and ensures that practice is standardised.
Challenges and barriers to implementing evidence-based practice
Resistance to change among healthcare professionals can be a significant challenge when implementing EBP yet there is little knowledge surrounding the factors that cause this resistance (Amarantou et al., 2018). Resistance to change is not new amongst nurses. Copnell et al., (2006) argued nurses were inherently resistance to change due to feelings of uncertainty, fear, distrust, frustration and confusion with Udod & Wagner, (2018) stating that resistance is an ordinary and predictable reaction to change. Organisational issues have been identified as a factor leading to resistance to change in particular poor communication within the organisation leading to uncertainty and unclear time frames for change implementation (Amarantou et al., 2018). Curtis and White (2002) investigated the fear of change being a threat to normal practices and the worry of the impact of that change on an individual’s ability to work within a new environment. Perceived lack of time and resources for training and reviewing latest research can also be barriers to implementing EBP. Implementing EBP requires a focused approach ensuing all team members are a part of this change. Lewin’s (1947) change theory recommends 3 stages; unfreezing, change and freezing. Informing staff and explaining what EBP is and the benefits it can have on patient outcomes assists with unfreezing. Using focused educational strategies and mentor support will help embed change with clear metrics and timely feedback to staff helping to embed the change into practice. There may still be resistance to the change, but this should be viewed as positive and an opportunity to review the level of success and to improve the change process (DuBose and Mayo, 2020).
Integral to embedding change is a positive and well-established culture of change, often nurtured by supportive leadership. In a culture that embraces change with supportive leadership, the focus is on aligning with new guidelines or EBP rather than repelling them.
How to implement evidence-based practice
Given the escalating difficulties associated not only with staffing but also with service demand, it is imperative to provide clinicians with the necessary tools and resources to effectively adhere to evidence-based practise (EBP) in a streamlined manner. A key factor in implementing EPB, to reduce unwarranted variation is the development of initiatives such as focused education; pathways supported by national guidance, and locally generated evidence, which can be implemented through an integrated whole-system approach using a Quality Improvement (QI) methodology to facilitate sustained improvements in quality. An integrated whole-system approach is essential in enabling organisations and teams to work together to respect other perspectives and create empowerment and cultural change that assists the effective collaboration to improve outcomes (Jones et al., 2021). QI methodology provides a structured approach that facilitates implementation and testing of change ideas, leading to service improvements and improved patient outcomes. QI can be used to implement EBP, combining the change with methods to achieve an outcome. There are good examples in the literature where the use of QI methods improved outcomes for patients with lower limb wounds (Dowsett and Taylor, 2018, Irvin et al., 2018). QI helps clinicians understand current process, design a new way of working, how to deliver and sustain the change. A PDSA (Plan, Do, Study, Act) cycle can support this method to adjust the approach throughout the process when required to achieve the intended improvement.
Clinical pathways
Clinical pathways can be a facilitator of change as they provide a seamless and easy to follow process for clinicians, which have the capacity to promote safe and logically oriented recommendations for the management of a specific condition whilst contributing to the reduction of complications and treatment errors (Kiyama et al, 2003) and promoting four goals:
- Decreased care fragmentation
- Optimised cost effectiveness
- Improved patient throughput
- Enhanced patient and family education regarding an anticipated treatment course (Dobesh et al, 2006).
Pathways can reduce variation in care by standardising practice and promoting patient safety, which subsequently contribute to improved clinical outcomes when compared with standard care processes (Hegarty et al, 2015). Clinical pathway implementation can align clinical practice with national guidance to provide high-quality care, which can assist with achieving cost effective practice (Adamina et al, 2011; Wounds UK, 2018). The National Institute for Health and Care Excellence (NICE) provides national guidance on specific health and social care needs that can be used in the development of clinical pathways to benefit patients, and carers, healthcare professionals, and organisations. NICE is a non-departmental public body that ensures the guidance meets regulatory bodies standards, through a rigorous and robust process, independently undertaking a thorough assessment of all the best available evidence, while considering implementation issues and overall cost effectiveness.
Therefore, NICE can empower all users to have confidence in their approach to care (Chidgey et al, 2007), additionally clinical pathways support managing patient expectations and encourage the patient to become engaged in their care (Cherif et al, 2020).
It is vital that clinical pathways are based on the most up to date evidence and national recommendations. Within wound care services it is often Tissue Viability leads or Specialist wound care services who develop and implement pathways within their own area. It is essential that these pathways are reviewed on a regular basis and updated in a timely manner to reflect new products and innovations. When the evidence base is established e.g. through NICE recommendation, it should never be a consideration of ‘should we make this change’ instead focusing on ‘how do we make this change’, as this is the only way that the true cost-effective benefits can be operationalised throughout the NHS.
Conclusion
Despite the wealth of empirical evidence in relation to the management of patients with VLU significant disparities in healthcare provision persist throughout the United Kingdom. There is a clear requirement to increase the use of evidence-based care and discourage the overuse of therapies for which there is insufficient evidence. There is an urgent need to improve the care of patients, ensuring time to healing is optimised through the use of evidence-based care. The burden of wound care on the NHS continues to grow year on year. NHS services need to turn away from merely managing wounds and instead truly focus on healing wounds. Access to evidence-based care is key to improving healing rates. Leaders of wound care services need to ensure there are EBP clinical pathways in place and that clinicians have easy access to evidence-based products. Ultimately it is the responsibility of the formulary owners to adopt evidence into practice, they need to be able to justify their decisions and be held accountable for how they spend public money. Where there may be challenges, e.g., ensuring every patient has consideration of venous intervention only through facilitating such access can health care areas effectively mitigate the unwarranted variation in care, resulting in enhanced healing rates, decreased risk of recurrence, and ultimately, an improved quality of life for patients.