The shortage of registered nurses in the UK is a cause for concern. NHS England and NHS Improvement (NHS vacancy statistics, 2022) data from 31 March 2022 shows a vacancy rate of 10.0% within the Registered Nursing staff group (38,972 vacancies), a slight increase from the same period the previous year when the vacancy rate was 9.2% (34,678 vacancies). This is worse in the community setting where the Nuffield Trust (2021) report that since 2009 there has been a significant reduction in the level of nurses in community health services with numbers declining by 45% between February 2010–February 2021.
This is of particular relevance to wound care as Guest et al, (2020) reported that in 2017/2018 68% and 85% of the costs for managing acute and chronic wounds, respectively, were incurred in the community. These figures demonstrate the importance of being able to provide evidence-based care and pathways for all staff to use to promote equity of care and to ensure timely and appropriate interventions. Furthermore, the reduction in staffing numbers impacts on the ability to deliver clinical care and attend educational sessions. Where there are staff shortages, care delivery is always prioritised and, as a result, education is cancelled.
The UK population is continuing to age, with the Office for National Statistics (2020) reporting there are approximately 11 million people aged 65 and over corresponding to 19% of the total population. They predict this will increase to almost 13 million people or 22% of the population by 2030. With the increasing age of the population and decrease in good health we can assume there will also be a decrease in skin integrity and people presenting with a range of complex wounds as identified by (Guest et al, 2017; Guest et al, 2020).
With the ageing population and increased demand for healthcare, along with potentially limited resources for education, there is an need to provide evidence-based, easy to access resources for staff.
The provision of evidence-based care is the cornerstone of nursing and in particular in wound care where rising costs (Guest et al, 2020) serve to reinforce the need for evidence-based care pathways that will improve patient outcomes (Matos, 2017)
Keeping up-to-date can be challenging for clinicians, with a plethora of articles, research and case studies as well as industry literature available. The advent of care pathways in the 1980’s (Rotter at al, 2021) derived from best practice statements, national guidance and research, which has enabled the delivery of evidence-based wound care for patients with defined diagnosis and symptoms (Rotter et al, 2021). Effective care pathways have the potential to improve care through standardisation (Lawal et al, 2016).
Clinical pathways for managing wound infection and biofilm
Clinical pathways provide a structured approach for health professionals to refer to when managing a range of healthcare interventions, including prevention, identification and management of wound infection while ensuring antimicrobial therapy is used appropriately.
Wound infection is the invasion of a wound by proliferating microorganisms to a level that invokes a local, spreading and/or systemic response in the host (International Wound Infection Institute [IWII] 2016; 2022). If a wound is not responding to standard protocols of care, in the absence of laboratory-confirmed diagnosis, best practice suggests that the presence of biofilm be presumed in wounds displaying signs and symptoms of chronic inflammation (Table 1). Furthermore, in a systematic review of the literate by Malone et al (2017) estimated over 75% of all non-healing wounds contain biofilm.
Evidence recommends that if a biofilm is suspected, debridement should be undertaken as an integral aspect of biofilm-based wound care (BBWC) and wound hygiene (Wolcott and Rhoads, 2008; Schultz et al, 2017). Biofilm can contain bacteria with genetic resistance to antibiotics as well as phenotypic tolerance to antibiotics as a consequence of being in the biofilm structure (Bowler, 2018). Biofilms form rapidly in wounds, with extensive regrowth demonstrated within 24–48 hours (O’Neill, 2014). Research has identified that biofilms can develop in acute and non-healing wounds deep within the extracellular matrix of slough, debris, necrotic and other tissues (Rhoads et al, 2008; Bianchi et al, 2016), as such it is evident that removing non-viable tissue via rapid debridement methods will disrupt and reduce biofilm (IWII, 2022). BBWC consists of wound debridement, wound cleansing, application of topical antimicrobials post-debridement and systemic antibiotics targeting the causative microbes (only if the wound is clinically infected, and under the supervision of antimicrobial stewardship; Malone and Swanson, 2017). The goals of therapeutic cleansing and debridement in BBWC have been described (Malone and Swanson, 2017; Schultz et al, 2017; Murphy et al, 2020) identifying the importance of physically removing the most virulent microorganisms from the wound bed while creating an environment that prevents or delays biofilm reformation.
There are numerous debridement methods available including surgical, sharp, conservative-sharp and mechanical methods, for example, monofilament, foam pads and ultrasonic debridement). The cleansing and combined antimicrobial action of UrgoClean Ag reduces bacterial load within the wound and continually cleans the wound, keeping the wound clean of exudate, slough and bacterial residue. This effectively reduces local infection and helps to reduce the amount of community nurse visits for dressing changes.
With the current healthcare staffing levels, reduction in resources and difficulties in securing time away from clinical areas for educational events, evidence-based pathways promote consistency of care and support staff when choosing interventions.
Development of a local biofilm pathway
In 2017 Leicestershire Partnership NHS Trust (LPT), published and implemented a biofilm pathway aimed at eradicating biofilm through a combination of mechanical debridement and antimicrobial dressings. The 2017 LPT biofilm pathway (Figure 1), although successful for some patients had a cohort that, despite destruction of biofilm and prevention of reformation, did not heal.
Reflecting on the 2017 implementation process of the pathway several barriers to success were considered (Table 2). To overcome these barriers the Clinical Lead Tissue Viability worked with industry partners to implement the new pathway that was underpinned by current research and evidence (Figure 2).
Before launching the updated pathway, it was important to acknowledge why implementation of the 2017 pathway had not been successful and to learn from this. Pathways need to not only inform healthcare professionals of best practice but also clearly demonstrate the value of improved patient satisfaction, increased healing rates, decreased caseloads, and decreased healthcare costs (Azevedo et al 2020; Wilde, 2020). Table 3 compares the 2017/2022 pathway launches.
Surfactants remain an intrinsic part of BBWCs due to their ability to penetrate slough/necrosis, reduce surface tension at the wound bed, therefore loosening any debris and break up of mature biofilm (Tyldesley at al, 2019). For this reason, the clinical lead for tissue viability stated that a surfactant soak was to continue to be used pre-dressing change ensuring biofilm in the deep tissue would be targeted.
Methods
We undertook an evaluation to identify whether UrgoClean Ag was effective at managing non-healing wounds via the pathway. UrgoClean Ag has polyabsorbent fibres that are suitable for use on wounds with signs of infection. The LPT tissue viability team undertook a patient clinical evaluation in 2020/21. Patient records were examined before the implementation of the pathway and then again after. We collected the data on wound size, duration, amount of slough and the number of the dressings used.
For qualitative feedback we asked those using and receiving treatment via the non-healing wound pathway for feedback on their experience.
Results following implementation of the UrgoClean Ag non-healing wounds pathway
The LPT tissue viability team undertook a 53 wound/19 patient clinical evaluation in 2020/21.
Before the implementation of the non-healing wound pathway, the average duration of wounds was eight months with daily dressing changes. Implementation of the new pathway led to a reduction in wound duration to 5.5 weeks, a 37.8% reduction in wound size and a 67% decrease in slough. There was an average of 3.17 dressing changes per wound per week.
Qualitative feedback
It was important to understand staff and patients’ perspectives of using the pathway. Evaluation data for the 2022 pathway included patient and staff feedback demonstrating the positive outcomes a structured non-healing wound pathway can have. We did not receive any negative comments. Community nurses reported:
- ‘Patient is happy…maceration is reduced and the ulcer is healing’
- ‘Pathway was easy to follow with the dressing process being easier leading to faster visits’
- ‘Reduced infection and pain following the updated pathway’
- Patient feedback was positive:
- ‘I couldn’t believe the difference (to the wound) in one week’
- ‘I had a chronic ulcer for 2 years and now it is healed!’
- ‘Can see the difference in what my wound looks like’
- Possibly the most poignant feedback highlighting benefits of an evidence-based pathway that all staff follow:
- ‘So grateful to be given this treatment’.
Case Study using the non-healing pathway
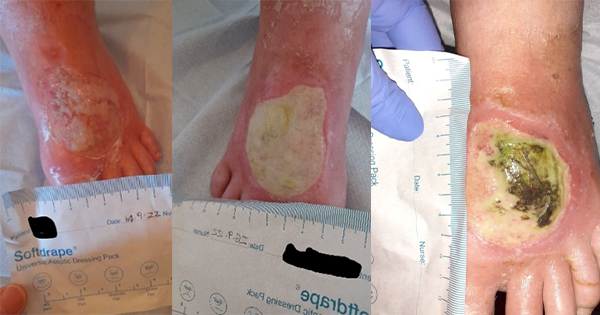
The updated 2022 pathway using UrgoClean Ag and a surfactant Prontosan (B Braun) was used to guide the care of a 72-year-old female with a past medical history of chronic obstructive pulmonary disease (COPD), hypertension, peripheral arterial disease and ischaemia. In June 2020 she had undergone an aorta bifemoral bypass following critical limb ischaemia to the right leg. In September 2022 a blister developed to her right foot (Figure 3A), which she was managing herself at home using cotton wool. She was admitted to the care of the community nursing team on 14 September 2022. The skin, foot and wound were assessed. The wound was noted to be superficial with fragile, oedematous surrounding skin, measuring 60mm x 60mm, 80% of the wound bed was covered in slough, 20% being granulation tissue. On a visual analogue scale of 0–10 (0 being no pain and 10 highest pain level) the patient reported her pain to 3. A silicone foam was prescribed to manage the wound.
After two weeks (Figure 3B) the patient was reassessed. The wound bed had deteriorated presenting as 100% slough. There were signs of excoriation on the periwound area with thin, serous moderate exudate, the wound size was unchanged. On assessment there were no clinical signs of infection, and the patient reported her pain remained at 3. A hydrofiber and silicone foam were applied to the wound at this time.
Following two weeks of treatment with the hydrofiber and silicone foam the patient reported increased pain, recorded as a 5, the wound bed continued to show clinical signs of deterioration (Figure 3C) and in conjunction with the wound care community nurse the decision was made to commence the patient on the non-healing wound pathway.
The patient and wound were reassessed following implementation of the pathway (Figure 3D). The wound bed showed improvement with 30% slough and 70% granulation tissue. There was also a reduction in the wound size to 53mm x 60mm x 1mm and the pain score was recorded as 0.
The wound was then reassessed twice a week and continued to improve and progress. Continuous use of UrgoClean Ag meant that by the 3 January 2023 slough greatly reduced with the wound measuring 50mm x 60mm x 1mm (Figure 3E). By 20 March 2023 the wound had almost healed displaying superficial areas of granulation tissue, no reports of pain or signs of clinical infection (Figure 3F).
Limitations
In terms of generalisability it should be noted that this pathway uses one antimicrobial dressing and surfactant. Furthermore this was a local small evaluation to establish local experience. Further clinical evidence can be reviewed in published literature.
Summary
The impact of reduced visits on community nurse caseloads cannot be underestimated. Guest et al (2020) stated the prevalence of wounds has increased by 71% in five years. This against the backdrop discussed earlier of dwindling nursing capacity, highlights the need for proactive care that harnesses emerging technologies in a easy to use and implement format, to standardise practice safely and effectively (Lawal et al, 2016; Mattos, 2017).
Implementation of an evidence-based pathway to manage non-healing wounds using topical advancements to eradicate infection and biofilm, is an important contributor to responsible antimicrobial stewardship (Edwards Jones et al, 2019; Edwards Jones, 2020). Guest et al (2020) highlight the increasing number of community nurse visits required for wound care against a backdrop of nursing shortages and increasing patient frailty and complexity (Carvalho, 2020). Clinical pathways can improve quality of care by translating multiple evidence sources for clinicians and turning them into an easy-to-follow process (Rotter et al, 2019). There are barriers to successful implementation at all levels and these need to be considered and addressed at the planning phase for a pathway to be successful. Early results in those areas that have successfully launched the non-healing wound pathway are promising, with progression to healing and reduced visits being seen in the initial cohorts. A collaborative approach to implementing a new pathway is essential with regular evaluation. Each pathway should be based on the best available evidence with feedback from patients.