This meeting report is based on a ‘Made Easy’ session that took place at the Wounds UK Annual Conference in Harrogate on 11 November 2025. The session and meeting report were supported by an educational grant from L&R.
The ‘Made Easy’ session aimed to demystify wound debridement by reviewing its current definition, clinical rationale, available methods, best practice considerations and key factors influencing technique selection. The session concluded with a hands-on demonstration, allowing delegates to trial a novel mechanical debridement technology designed to support effective and user-friendly wound bed preparation.
Acute wound healing typically progresses through four overlapping phases over 4 to 6 weeks. These stages are haemostasis, inflammation, proliferation and maturation/remodelling (Wallace et al, 2023). When this orderly sequence is disrupted or fails to progress in a timely manner, wounds may become chronic. Chronic wounds (also known as non-healing wounds) are associated with delayed healing, increased pain burden and a higher risk of complications for patients, as well as placing an increasingly high burden on healthcare systems (WUWHS, 2019; Tettelbach et al, 2022).
There are many factors that can hinder the wound healing process and increase the likelihood of a wound becoming chronic. Key contributors include low oxygen levels (hypoxia), the presence of microorganisms such as bacteria, reduced blood flow (ischaemia), and factors affecting collagen synthesis such as diabetes and malnutrition (Wallace et al, 2023).
Regular and comprehensive debridement, defined as the removal of non-viable or foreign material from a wound, serves as a foundational element in wound care. Various papers demonstrate that optimal debridement practices significantly enhance the rate of wound closure and reduce the risk of infection (Steed, 2004; Wilcox et al, 2013; Ousey et al, 2025). In a retrospective study analysing data from two randomised clinical trials including 366 venous leg ulcers and 310 diabetic foot ulcers, the authors concluded that in these patients, surgical debridement was more effective than no debridement in improving closure rates, as evidenced by a more significant reduction in wound surface area over 12 weeks (p=0.019). It was also suggested that debridement frequency may play a role in improving healing rates (Cardinal et al, 2009). While a range of debridement methods are currently used in practice, healing rates improve regardless of the method, as seen over a wide range of chronic wounds, and especially when performed on a frequent basis (Wilcox et al, 2013). Despite this evidence, debridement remains underutilised in practice (Ousey et al, 2025).
What is debridement?
Debridement is defined as the removal of viable and non-viable wound components, including necrotic tissue, slough, microorganisms, biofilm, extracellular polymeric substance (EPS) and foreign material, using the most effective method with the fewest side effects (WUWHS, 2019; Mayer, 2024). By removing these barriers to repair, debridement can help re-initiate the wound healing process and reduce the overall duration of care (Strohal et al, 2013; Mayer, 2024).
Some wound components, such as necrotic tissue and slough, are readily visible and can be identified through routine clinical assessment. In contrast, microorganisms and biofilm are not directly visible at the point of care, yet are estimated to be present in most chronic wounds (WUWHS, 2019; Mayer, 2024). Their presence is often inferred from indirect clinical indicators, including stalled healing, recurrent inflammation or a lack of response to antimicrobial therapy.
Although advanced diagnostic tools, such as fluorescence imaging and ultraviolet blotting, can assist in identifying microbial burden, best practice guidance recommends that clinicians should assume the presence of microorganisms and biofilm in chronic wounds and address them as part of routine cleaning and debridement (WUWHS, 2019; Li et al, 2021; Tettelbach et al, 2022).
Why do we debride?
Debridement is an essential component of wound management (IWII, 2022). Devitalised tissue acts as a multifaceted barrier to wound healing. Physically, it obstructs the migration of epithelial cells and fibroblasts, all of which are essential for granulation and re-epithelialisation (Mayer, 2024). When left in situ, devitalised tissue creates a stagnant wound environment that prevents healthy tissue formation, delays wound closure and increases the risk of chronicity (Tettelbach et al, 2022). Its presence is also associated with increased exudate production, reduced penetration and efficacy of topical therapies, damage to surrounding skin, malodour and an increased risk of bacterial proliferation and infection (Fletcher, 2008; Tettelbach et al, 2022; Mayer, 2024).
Beyond its physical effects, devitalised tissue sustains chronic inflammation by perpetuating the release of pro-inflammatory cytokines and mediators. This prolongs the inflammatory phase of wound healing, leading to persistent swelling, pain and exudate. Although immune cells such as macrophages remain activated, their function becomes ineffective, contributing to further tissue damage and ongoing necrosis. In the absence of adequate debridement, wounds can become trapped in this inflammatory state, increasing patient discomfort and complicating management (WUWHS, 2019; Li et al, 2021; Tettelbach et al, 2022; Mayer, 2024).
Devitalised tissue also provides a nutrient-rich substrate for microbial growth, supplying proteins, lipids and other elements that support bacterial proliferation within the wound bed (WUWHS, 2019; Mayer, 2024). This elevated bioburden increases the risk of local infection and, in severe cases, systemic complications such as sepsis (White et al, 2015; Mayer, 2024). Regular debridement interrupts this nutrient supply, reducing bacterial load and supporting a more favourable healing environment (Mayer, 2024). In addition, devitalised tissue facilitates the formation and persistence of biofilm by offering a protected environment in which bacteria can become encased within an EPS (WUWHS, 2019; Mayer, 2024). Biofilms, reported to be present in the majority of chronic wounds, are highly resistant to host immune responses, antibiotics and topical antimicrobials, allowing bacterial communities to persist and rapidly re-establish following incomplete removal (WUWHS, 2019; Tettelbach et al, 2022; Mayer, 2024). Targeted debridement combined with effective cleansing disrupts these structures, enabling stalled wounds to progress along a healing trajectory (WUWHS, 2019; Mayer, 2024).
The workshop further highlighted the importance of debriding beyond the wound bed to include the wound edges and surrounding skin. Contaminants and devitalised tissue frequently extend into the periwound area, where features such as hyperkeratosis or callus can impede healing (FDUK, 2014; WUWHS, 2019; Mayer, 2024). International guidance recognises the periwound region as extending well beyond the visible wound margin, emphasising the need for thorough assessment and cleansing of these adjacent tissues (WUWHS, 2019; Mayer, 2024; IWII, 2025).
Crucially, debridement should be viewed as an ongoing process rather than a single intervention. It is unlikely that all devitalised tissue and debris will be removed in one session; therefore, repeated debridement is required until the wound demonstrates clear signs of progression towards healing (Wilcox et al, 2013; Tettelbach et al, 2022; Mayer, 2024). Despite this, debridement remains inconsistently applied in practice, with approximately one quarter of wounds not debrided adequately and only a minority receiving weekly debridement, underscoring a persistent gap between evidence-based recommendations and real-world care (Wilcox et al, 2013; Tettelbach et al, 2022).
What do we debride?
Debridement targets both visible and non-visible components that act as barriers to wound healing [Table 1]. These elements may be present within the wound bed, at the wound edge or in the surrounding tissue and their effective removal is essential to restore a physiological healing environment (Tettelbach et al, 2022; Mayer, 2024).
Visible targets for debridement include necrotic tissue, slough and foreign material. Necrotic tissue consists of dead or devitalised tissue, often presenting as black or brown, dry, leathery eschar which can interfere with wound contraction. In most cases, removal is necessary to reduce infection risk and enable granulation and re-epithelialisation. However, in individuals with arterial insufficiency, caution is required; current guidance advises that stable, dry necrotic tissue should not be debrided unless there is evidence or suspicion of infection beneath the eschar (FDUK, 2014; Mayer, 2024).
Slough is commonly observed in chronic wounds and typically appears yellow or white, with a stringy or fibrinous texture. It is composed of exudate proteins, degraded extracellular matrix components, inflammatory cells and microorganisms. Slough may be loosely adherent or firmly attached to the wound bed, but in all cases the removal of slough is important to allow the wound to form granulation tissue and re-epithelialise (WUWHS, 2019; Mayer, 2024). Furthermore, components such as slough and necrotic tissue can also prevent permeation of antibiotics into the wound cavity by acting as a mechanical barrier, while simultaneously promoting bacterial growth and biofilm formation (Steed, 2004; Kaiser et al, 2021).
Foreign materials, such as debris, fibres or retained sutures, are also usually visible and should be removed to prevent prolonged inflammation or infection. Selective debridement techniques are required to protect viable tissue and underlying structures (Tettelbach et al, 2022; Mayer, 2024).
Biofilm, defined as a structured community of microorganisms encased within an EPS, is widely recognised as a major impediment to healing in chronic wounds (WUWHS, 2019; Mayer, 2024).
Both biofilm and EPS contribute to antimicrobial tolerance and sustained inflammation, allowing bacterial communities to persist despite treatment.
How do we debride
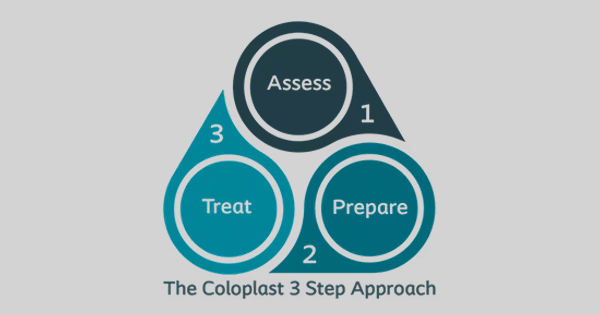
Cleansing is the essential first step in the debridement process. Its primary aim is to reduce bacterial bioburden and remove surface contaminants, debris and microorganisms, establishing a clean environment that supports wound healing. Thorough cleansing before and after debridement improves wound bed visualisation, reduces the risk of biofilm reformation and contributes to better clinical outcomes (IWII, 2025). Cleansing can be performed with inert solutions (such as saline, sterile water or non-sterile tap water), surfactants and antiseptics (IWII, 2025). While cleansing and debridement are separate steps in wound bed preparation, they both contribute to the removal of wound components such as slough or foreign materials and should therefore be treated as equally important in the wound management pathway (Ousey et al, 2025).
Removal of unhealthy surrounding skin, often referred to as skin hygiene, is also a key component of wound bed preparation. Dry, macerated or hyperkeratotic skin can harbour bacteria capable of invading the wound and delaying healing (WUWHS, 2019; Mayer, 2024). Whether classified as debridement or therapeutic cleansing, this process should always be performed with deliberate intent and care (Tettelbach et al, 2022; Mayer, 2024).
Debridement methods include sharp, autolytic, larval, enzymatic, and mechanical approaches (LeBlanc et al, 2024; Ousey et al, 2025). Mechanical debridement is a commonly used method, notable for its speed (often completed within minutes) and minimal invasiveness, particularly when using modern products. It also does not require specialist skills, making it accessible to most clinicians, and is suitable for the preparation of complex chronic or infected wounds. The process involves physically removing devitalised tissue, slough and biofilm from both the wound bed and the periwound area using specialised pads or tools. Regular maintenance debridement, combined with thorough cleansing, prevents stagnation and supports ongoing healing (FDUK, 2014; WUWHS, 2019; Mayer, 2024).
Patient comfort, tissue fragility and clinician skill should be considered when selecting debridement methods, but these factors should not prevent routine intervention. Pinpoint bleeding is generally accepted as an indicator of reaching viable tissue. Overall, consistent, effective cleansing and debridement practices maintain a healthy wound environment and promote healing (Mayer, 2024).
Factors influencing choice
The choice of debridement method is influenced by multiple factors, including clinical need, clinician experience and competency. For example, all clinicians can perform autolytic and mechanical debridement, while sharp techniques should be reserved for those with advanced training (FDUK, 2014; LeBlanc et al, 2024; Mayer, 2024). Other factors include urgency of tissue removal, level of inflammation, local accessibility, patient age, presence of infection, risk of exposing underlying structures, treatment objectives, wound type and depth, treatment setting and patient preferences (Wilcox et al, 2013; Mayer, 2024).
Clinical cautions
Prior to initiation of any form of debridement, the healthcare clinician must first conduct a comprehensive, holistic patient assessment (LeBlanc et al, 2024), particularly as debridement should be approached with caution in specific situations. For example, necrotic tissue should not be removed in patients with severe peripheral arterial disease (critical limb ischaemia) who are not candidates for revascularisation (FDUK, 2014; Mayer, 2024) or in patients with inflammatory or immune-mediated conditions such as pyoderma gangrenosum, as debridement may exacerbate tissue damage. Clinicians should also consider the location of slough relative to deep structures such as vessels, nerves, tendons, fasciaa or muscles, as well as the patient’s overall condition, medications and the clinician’s skill and expertise (WUWHS, 2019; LeBlanc et al, 2024; Mayer, 2024).
Introducing a new side to mechanical debridement
Building on user feedback, L&R introduced Debrisoft® Duo [Figure 1], a dual-sided monofilament fibre pad designed to enhance mechanical debridement (Morris, 2018; Schultz et al, 2018; NICE, 2019). The original soft white side efficiently removes debris, exudate, slough and biofilm from the wound bed, while the new textured beige side targets firmly adherent, fibrinous devitalised tissue and components which are more tightly adhered. Together, this 2-in-1 design supports efficient, consistent debridement across a range of wound types (Head et al, 2025).
Debrisoft® is currently the most commonly used mechanical debridement pad in the NHS (IMS data, 2026) and has demonstrated effectiveness in disrupting and binding biofilm (Schultz et al, 2018; Mayer, 2024). It is suitable for use across a range of wound types and patient characteristics and does not require specialised skills (Stephenson et al, 2016; NICE, 2019; Mayer, 2024). Its use is supported by NICE guidance (NICE, 2019).
Debrisoft® removes both visible slough and non-visible bacteria/biofilm to promote wound healing, with in vitro studies confirming effectiveness in all cases (Morris, 2018; Schultz et al, 2018; Mayer, 2024). This is demonstrated in Figure 2 (Morris, 2018) using fluorescence imaging, where there is a clear reduction in bacterial burden following debridement with Debrisoft® (as seen by a reduction in red fluorescence following debridement). Furthermore, an audit of 486 patients with unhealed wounds showed that incorporating regular debridement with Debrisoft® led to a 43% reduction in patients requiring ongoing wound care prescriptions, a 14% reduction in overall prescription costs and a 33% reduction in antimicrobial expenditure, emphasising the significant clinical and economic benefits associated with its regular use in wound management practice (Burnett et al, 2021).
Conclusion
This Made Easy workshop reinforced that debridement is not just an occasional intervention, but a fundamental and ongoing component of effective wound bed preparation. By clearly defining what should be removed, why removal is necessary and how debridement can be safely and efficiently performed in routine practice, the session helped to normalise and demystify debridement across clinical settings. The hands-on demonstration further highlighted how modern mechanical debridement technologies can support confident, consistent practice by a wide range of clinicians, enabling thorough cleansing and targeted removal of devitalised tissue without the need for advanced competencies. Taken together, the session emphasised that regular, well-executed debridement, combined with effective cleansing, remains key to overcoming wound stagnation, reducing microbial burden, and supporting progression towards healing in chronic wounds.