In the spring of 2016 the NHS, funded by the Department of Health, commissioned a Clinical Evaluation Team hosted by the NHS Business Services Authority with a remit to provide an independent clinical review of everyday healthcare consumables. This followed Lord Carter’s review, which suggested, transformation was required in the development of national category standards. The commissioning of this piece of work was based on the lack of clinical standards available and lack of clinical review on a national basis.
In October 2018 the clinical review of foam dressings was published, providing a clinical assessment of the usability and requirements from the NHS for foam dressings. It also contained a clinical statement of the desired functions and properties that health care professionals require.
The CET report referenced 2016 data from the NHS Supply chain that Trusts were purchasing over 10.5 million foam dressings annually, a figure that has been increasing year-on-year with an annual spend of £25 million.
Following an extensive consultation and clinical engagement, products were evaluated based on agreed clinical criteria that considered; packaging, opening and preparation for use, clinical use, and disposal. The products included in the review were also assessed and ranked on their level of evidence.
The rating in the CET report for each aspect scored a product from 0 to 3 where 0 does not meet the criteria, 1 partially meets the criteria, 2 meets the criteria and 3 exceeds the criteria. The numerical scores across all measures were totalled and a mean value given. Criteria with a yes/no response were converted into aggregate percentages and star ratings assigned.
This CET report could then be used by organisations across the country to help support them when making formulary decisions. However, although a numerical score was provided there was no clear conclusion as to which product performed the best in clinical practice leaving health professionals to draw their own conclusions.
Doncaster Skin Integrity Team
Doncaster and Bassetlaw Teaching Hospitals Foundation Trust (DBTH), serves a population of more than 420,000 across South Yorkshire, North Nottinghamshire and surrounding areas. DBTH was one of the first 10 trusts in the country to achieve Foundation Trust status in 2017 and is one of only five Teaching Hospitals in the Yorkshire region, training around 25% of all medical students in the region and 30% of all other health professionals. DBTH is one of the largest employers in the local area employing around 6,600 members of staff, around 3,000 of which are clinical. The Skin Integrity Team consists of a team of experienced professional and support staff, to provide specialist care, support and education to healthcare professionals, patients and carers who are preventing and managing a wide range of wounds.
Evaluation of silicone foam adhesive dressings
Following the publication of the CET report on foam dressings the Doncaster Skin Integrity Team used the detail within the report, to support a further local evaluation to select the product that would best meet their population needs. This evaluation was carried out between August 2019 and June 2021.
As silicone foam adhesive dressing was the product category most used across the local health community, the Lead Nurse for the Skin Integrity Team chose to focus on this product range to draw further clinical conclusions to support a formulary listing. This product usage also mirrors the findings from the CET report which highlighted that foam dressings are one of the most widely used dressings.
Within the CET report a set of clinical criteria were defined in order to evaluate each the products that were represented from a large range of brands and suppliers available through the NHS National procurement providers framework.
To simplify the data for translation into clinical practice, seven ‘Clinical Use Criteria’ (Table 1) were selected from the comprehensive list within the CET report. These were identified as being the most significant and this was developed into a scoring system, referred to as the ‘Vernon Scoring System’ to support the local evaluation. Figure 1 describes the evaluation timelines.
The top six silicone foam dressings were then identified (Table 2) for inclusion in the local evaluation. A decision was made during the evaluation period to remove the UrgoTul Absorb Border dressing from the evaluation, as this has a Lipido-Colloid contact layer, which is not a component in any of the other Silicone Adhesive Foam dressings and inhibits the use of other products used in combination with the silicone foam adhesives.
The Vernon Scoring System provides a numerical ranking score with the total points gained receiving a ranking of 6 for the best performing, and 1 for the least performing. The Lead Nurse and the Skin Integrity Team systematically reviewed the star ratings within the CET report against the Vernon Scoring System and ranked accordingly (maximum 42 points; Figure 2).
Aim of the evaluation
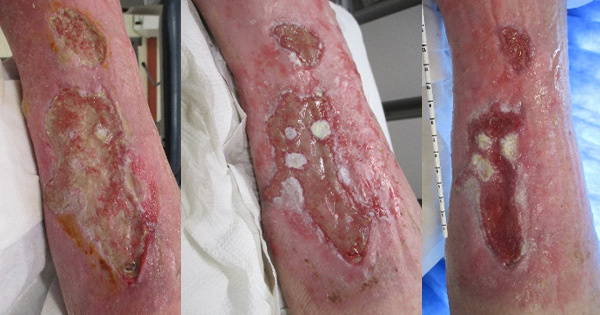
The aim of the local evaluation was to consider if a better-informed choice of silicone foam adhesive could be made, following a structured evaluation of the foam products in clinical practice. The authors decided to evaluate each different silicone foam dressing on 50 different wounds. When a patient had more than one wound, each wound was evaluated separately. Wounds were only monitored for the duration of the evaluation.
Evaluation method
The evaluation was undertaken in the clinical areas in Doncaster covered by the Skin Integrity Team who provided support and motivation for the nurses engaged in the evaluation. Ethical approval was not required, as this was an evaluation of wound dressings, which were already available in the area. All dressings used during the evaluation were provided by the different manufacturers.
Each silicone foam dressing was evaluated separately, therefore, once 50 wounds had been allocated to the first silicone foam and evaluation completed, then the team would start recruiting a further 50 wounds to the next silicone foam and so on (250 wounds in total).
All patients selected were asked for their verbal consent to be included and all had a full wound assessment following the National Wound Care Strategy Programme (NWCSP) minimum data set (MDS) for wound assessment (Coleman et al, 2017) to ensure their wound was suitable to treat with a silicone foam adhesive dressing. Patients meeting the criteria were provided with details of the evaluation, the rational for undertaking it and their role within the evaluation.
All clinicians had undertaken training in wound assessment and were provided with details of the project and the dressings included to ensure they understood in what capacity the dressings could be used. They were also provided with instructions on how to complete the evaluation form.
The data captured on the forms included details of the patients’ medical history, current medication, and allergies, along with gender and age. The general wound details included: wound type, location, measurement (width, length, and depth) and wound duration along with the primary and secondary dressing to be used and the frequency of planned dressing changes.
The rest of the evaluation form aimed to elicit the actual wound assessment and covered tissue type, recorded as percent of epithelialisation, granulation, slough, necrosis, signs and symptoms of infection, which included the opportunity to record an abnormal wound swab and organism cultured, and any current antibiotics. The level and type of exudate was included, as was information describing the surrounding skin including healthy, hyperhydration, maceration, erythema, dry and the presence of oedema.
The parameters describing the wound assessment were captured at each dressing change and the patient’s level of pain at dressing change was also recorded on a Likert scale where 0=no pain and 10=worst possible pain. Some other key information collected at dressing change elicited details to rate how well the dressings performed against the five clinical criteria (Table 3).
Conformability
- How easy the dressing was to apply, rated using a Likert scale of 0 to 10 with ten being the best
- Is the dressing an appropriate size and shape for the anatomical location? Response was yes or no, where no indicated the dressing was not conformable
- Were modifications required to keep the dressing in place? Response was yes or no, where yes was deemed not conformable
- Did the dressing stay in place? Response was yes or no, where no was deemed not conformable
- Did the wound require a filler or packer?
Absorbency and Fluid Capacity
- Was the dressing managing the exudate? Response was yes or no, where no deemed the dressing not sufficiently absorbent
- Number of days between dressing changes, selecting 1, 2, 3, 5, or 7 days. Dressings changed every 3, 5 or 7 days were deemed to be absorbent.
Moisture Vapour Transmission Rate (MVTR)
- The effectiveness of the MVTR was assessment by rating the condition of the periwound skin. If this was hyperhydrated and macerated, then MVTR was considered poor.
Pain on removal
- Did the patient experience pain on removing the dressing? Response was yes or no
- What was the pain score? Rated using a Likert scale of 0 to 10 where 10 was worst possible pain.
Ease of Removal
- Did the dressing remove in one piece? Response was yes or no, where no was deemed not easy to remove
- On removal was any periwound skin damage experienced? Response was yes or no where yes was deemed not easy to remove
- On removal was any wound bed damage experienced? Response was yes or no where yes was deemed not easy to remove.
- The evaluation data did not contain any patient identifiable information and thus maintained patient confidentiality.
Results
A total of 250 evaluations (50 for each different foam dressing) were undertaken. The most frequent recorded wound aetiology was surgical wound but other wound aetiologies recorded included pressure ulcers, leg ulcers, foot ulcers, donor/graft sites, abscess, and trauma wounds (Figure 3).
Most anatomical locations were reported but the most frequent recorded were wounds on the torso, the foot, toe and leg areas, followed by the pelvic and sacral areas (Figure 4).
The different silicone foam dressings were rated against the clinical criteria using the Vernon Scoring System and the results presented as a percentage (Figure 5). For dressing conformability Biatain Silicone foam was rated highest at 86%, the lowest score in this category was 65%. For absorbency and fluid handling Biatain again was rated the highest at 99%, the lowest rated score was 82%. For moisture transmission rate Allevyn Gentle scored 98%, the lowest score was 78%. For pain on removal of the dressing, Allevyn Gentle was rated highest with 95% as pain free, the lowest recorded in this category was 82% pain free. There was little difference between the products for ease of dressing removal and the scores ranged from 96% to 99%.
Following the aggregation of all the ratings a ‘Vernon Score’ was allocated to each dressing (score of 5 for the best performing and 1 for the least performing with a potential maximum of 30 points). Biatain Silicone and Allevyn Gentle scored equally at 24. This score was then combined with the CET evaluation ratings for each product by applying 5 points to the best performing dressing and 1 point for the least performing, giving a maximum of 10 points to one of the dressings. Following this Biatain Silicone was rated highest with an overall score of 10 (Figures 6–7).
Testimonials
‘’Due to the ability to use on wounds up to 2cm deep as a primary dressing, it cuts down on nursing time and cost as a cavity filler is not required. The simplicity of being able to use Biatain without a cavity filler has promoted simpler self-care for patients’’ – Toni Plumb, Skin Integrity Specialist Sister at Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust
‘’It has been easier to encourage patients to manage their own wounds due to the ease of application of Biatain. It has allowed patients to be more in control of their own wounds which has given them more freedom and allowed them to be less tied to hospital appointments’’– Gemma Long, Skin Integrity Specialist Support Worker.
Conclusions
Formulary decision making can be challenging to ensure that the most cost and clinically effective product is selected. The aims of the CET was to support this process but there remained some choice, by undertaking further clinical evaluations this has enabled the Doncaster Skin Integrity Team to be confident in their choice of the Biatain Silicone Foam. This was introduced to all clinical areas following the evaluation.