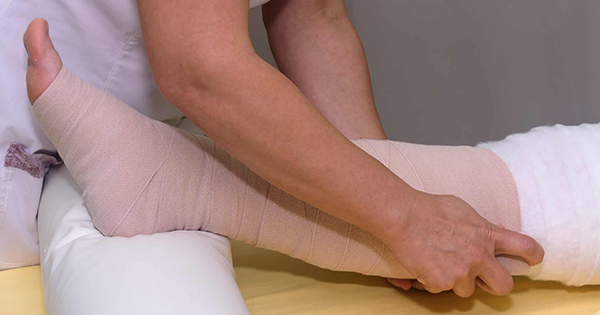
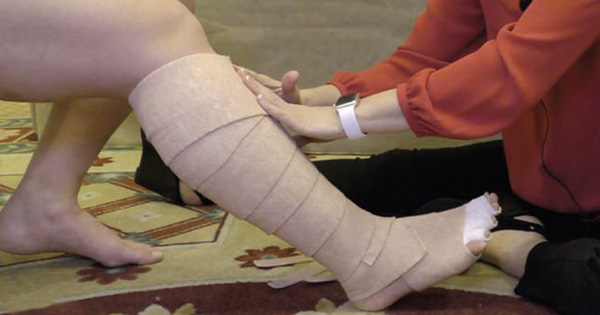
Chronic heart failure (CHF) is a complex clinical condition with increasing prevalence due to increasing rates of cardiovascular disease and the growing age of the general population (Urbanek et al, 2020). CHF has been referred to as a global pandemic, affecting at least 26 million people worldwide, and is increasing in prevalence, adding to the burden on healthcare systems and affecting patients’ quality of life (Savarese and Lund, 2017). The prevalence of HF depends on the definition applied, but is approximately 1–2% of the adult population in developed countries, rising to ≥10% among people >70 years of age (Ponikowski et al, 2016). The lifetime risk of HF at age 55 years has previously been stated as 33% for men and 28% for women (Bleumink et al, 2004).
In basic terms, heart failure means that the heart is unable to pump blood around the body efficiently; although this cannot be cured, it can often be managed, through lifestyle changes, medication, medical devices (e.g. a pacemaker), or surgery.
Along with breathlessness and fatigue, swollen legs and ankles can be one of the first signs of CHF (NHS, 2022). Patients with initial presentation of acute CHF may present with swelling in both legs that extends onto the trunk, usually accompanied by breathlessness on exertion or when lying flat (Evans and Ratchford, 2016). Symptoms can develop quickly (acute heart failure), or gradually over weeks and months (NHS, 2022). Acute presentation would generally be treated with physical means such as diuretics and vasodilators, plus making sure that treatment through medication is optimised, which would be followed by discussion of chronic oedema and ongoing treatment options.
Oedema is one of the fundamental features of heart failure, but the pathophysiology of oedema varies (Clark and Cleland, 2013). Patients present along a spectrum ranging from acute pulmonary oedema to gross fluid retention and peripheral oedema. In patients with pure pulmonary oedema, the patient does not have excess fluid, but pulmonary venous pressure rises so that the lymphatic system is unable to drain away the fluid. Conversely, in patients with peripheral oedema, the problem is one of fluid retention; therefore, removing the fluid is the most important consideration (Clark and Cleland, 2013).
Heart failure often only affects the left or right side of the heart, but can affect both. The three types of heart failure can be differentiated accordingly (NIH, 2018):
- Left-sided heart failure: The left ventricle of the heart no longer pumps enough blood around the body. As a result, blood builds up in the pulmonary veins. This causes shortness of breath, trouble breathing or coughing – especially during physical activity. Left-sided heart failure is the most common type.
- Right-sided heart failure: The right ventricle of the heart is too weak to pump enough blood to the lungs, which causes blood to build up in the veins. The increased pressure inside the veins can push fluid out of the veins into surrounding tissue. This leads to a build-up of fluid in the legs, or less commonly in the genital area, organs or the abdomen.
- Biventricular heart failure: Both sides of the heart are affected. This can cause the same symptoms as both left-sided and right-sided heart failure, such as shortness of breath and a build-up of fluid.
Overview of oedema
A survey carried out in 2012 estimated that, within the general population of the UK, approximately 3.99 people in every 1000 have chronic oedema (Moffatt et al, 2017). This is almost three times the previously reported prevalence of 1.33 per 1000 (Moffatt et al, 2003) and is thought to correlate with the increasingly ageing population and associated poly-morbidity. In people aged over 85 years, the prevalence of chronic oedema increases to 12 people in every 1000 (Moffatt et al, 2017). Additionally, CHF is a major risk factor for leg oedema, putting patients at elevated risk of oedema and associated complications.
Those with oedema are also at increased risk of developing a wound, and subsequently the wound becoming chronic or developing further complications. See Box 1 for some examples of additional risk factors associated with oedema. Moffatt et al (2019) reported that between 52% and 69% of patients cared for by community nurses had chronic oedema, and of these, 73% also had a leg ulcer.
An earlier study found that of 53% of patients with chronic oedema suffered an incident of cellulitis, of which 9% required hospital admission, with an average length of stay of 11 days being the average length; inappropriate referrals were also found to cost the district nurse an average of 5 hours per week (Moffatt and Pinnington, 2012).
Leaking lower limbs is a key concern, with an estimated 33% of lower limb patients having wet or leaking legs; therefore, reducing the number of weekend and out-of-hours visits made by community nurses to tend to patients with leaking or wet legs has been identified as a key aim (Bradford and Rossiter, 2020).
Oedema and CHF
Additional factors may also add to the risk of oedema for patients with CHF, including (Cooper, 2011):
- Limited mobility
- High BMI or malnutrition
- Some medications
- Venous insufficiency.
In patients with right-sided HF, blood stasis and venous and lymphatic hypertension result in swelling and fluid collection below the heart level (including in the lower limbs and sacral region or pleural cavity); in left-sided HF, a decrease in cardiac output, in addition to the possible subsequent influence on right-side heart function impairment, also activates compensatory (nervous and hormonal) mechanisms that result in peripheral oedema related to the activation of the renin-angiotensin system, antidiuretic hormone release, and retention of sodium and water (Urbanek et al, 2020).
The importance of compression
Although compression therapy is known as the gold-standard treatment for chronic oedema and lower-limb wounds or leaking lower limbs this is not routinely offered to patients with oedema due to CHF. This is often due to fear and misunderstanding on how much compression can be applied to a patient with heart failure, due to the lack of evidence on this topic. More evidence is needed and work needs to be done to dispel the myths that compression therapy is not suitable for patients with CHF, which are often based on fear of overloading the heart with too much fluid from the lower-limb veins once compression therapy is instigated.
However, recent research has shown that compression therapy is not a risk factor for the majority of patients with CHF and can in fact be used safely in selected CHF patients – i.e. stable CHF patients without decompensated heart function (NYHA I and II; Urbanek et al, 2020). Recent evidence suggests that initiating compression therapy in patients with stable and compensated heart failure is safe if appropriate precautionary measures are undertaken; however, there is still insufficient evidence to support the safety of compression therapy in patients with severe and decompensated heart failure and more guidance is needed for clinicians in this area (Saucedo et al, 2021).
Without compression therapy, this cohort of patients are often left with their only treatment being a superabsorbent dressing. This approach, based solely on ‘mopping up’ fluid rather than tackling the underlying cause through compression therapy, leaves the patient at further risk of skin damage and recurring lower-limb wounds (Anderson, 2017).
The use of compression therapy can be considered in stable CHF patients without decompensated heart function for both CHF-related oedema treatment and for treatment of the concomitant diseases leading to leg swelling occurrence (Urbanek et al, 2020). The use of MC in more severe classes of CHF (NYHA III and IV; Figure 1) should be the subject of future clinical studies to select the safest and most efficient compression method as well as to select the patients who benefit most from this kind of treatment.
A gap in knowledge
There is currently a lack of guidance around use of compression therapy in patients with oedema due to CHF. This lack of guidance has resulted in a gap in knowledge for clinicians, meaning that clinicians may be unable to confidently treat patients with oedema due to CHF.
However, it is important to note that clinical best practice documents state that there is very little risk of patient harm associated with compression, regardless of comorbidities. The National Wound Care Strategy Programme (NWCSP) recommends that all people with leg wounds should be treated with mild compression and that compression should be applied as early as possible (NWCSP, 2020). Unless specified ‘red flags’ are present, the benefits of first-line mild compression have been found to outweigh the risks, even for people without obvious signs of venous insufficiency (BLS, 2019).
The importance of compression therapy in appropriate patients cannot be overstated: without compression therapy, oedema in the legs will not improve, and the leakage is likely to increase, resulting in ulceration.
Working together
The current landscape means there are inconsistencies in care for patients with oedema, particularly when this is caused by CHF. A unified approach is needed to ensure that all patients with oedema receive optimal care.
The authors have identified a gap in care for heart failure patients, who often suffer from chronic oedema and leaking lower limbs. There is a recognised need to manage the underlying causes, rather than to treat resultant oedema and wounds solely using superabsorbent dressings. There is a need to combine specialist knowledge in CHF and compression therapy, in order to address this gap in knowledge, and to optimise care and outcomes for patients.
This currently represents a gap in care on a national scale and action is needed. The primary need is to dispel the myths and fears around compression for patients with CHF, and to provide clinicians with guidance on how this can be implemented safely.
Care pathway
To bridge this gap, it was decided a decision pathway would be beneficial in order to safely assess a patient for signs of acute decongestive heart failure and what treatment should be used depending on the patient’s condition. Following the National Wound Care Strategy guidance and Chronic Oedema Best Practice Statement, a draft pathway was formulated (Figure 2).
The pathway is intended to ensure that appropriate care is given to all patients with oedema, including compression therapy for patients with CHF where this is appropriate. The pathway is also designed to increase clinician confidence and provide guidance on clinical decision-making so that all appropriate care options are considered.
Development of the pathway
The pathway was developed with clinical decision-making in mind, addressing the questions that may affect the approach taken towards compression therapy for the individual patient. Red flags are considered that would contraindicated compression or mean that a more cautious or staged approach is required.
Before compression therapy is commenced, the pathway emphasises that the patient should be assessed for evidence of:
- Acute cellulitis
- Bilateral legs affected by oedema
- Soft pitting oedema.
Evidence of any of these means that a staged approach should be taken to compression, with an ongoing red flag assessment care plan implemented for the individual patient. The full pathway and the considerations that should be made can be seen in Figure 2.
Next steps
It was agreed that the scale of this problem means that more education is needed to enable clinicians to provide compression therapy to patients with CHF and to address disparities in care for people with oedema. The pathway is the initial step towards best practice guidance, which is currently being developed and will be published by Wounds UK in 2023.