This report is based on the presentation by Maureen Bates and Dr Prash Vas at the new Tech Symposium in the European Wound Management Association 2024 conference in London, United Kingdom. The speakers presented the trial design and preliminary data from their ongoing clinical evaluation aimed at assessing the effectiveness of antibiotic-loaded calcium sulphate (STIMULAN®) treatment in people with diabetic foot infections (DFIs) and complex wounds. The pathway outlined in this publication can be adapted as per local guidelines.
Calcium sulphate applications in diabetic wound management
Due to dysfunctional immune, metabolic and nervous system in even well-controlled diabetes, approximately 25% of all people living with diabetes develop a diabetic foot ulcer (DFU) over their lifetime (Singh et al, 2005; Mauricio et al, 2020). Bone deformity and peripheral arterial disease (PAD) increase the risk of DFUs in people with diabetes; furthermore, approximately 60% of all DFUs are infected upon presentation, posing significant danger to foot integrity and patient’s life (Crowther et al, 2021).
Due to the complications associated with diabetic foot and its vulnerability to infections, improving patient outcomes requires a targeted and multifactorial management approach. When treating an infected DFU, surgical debridement is undertaken to remove infected areas, including soft tissue and deformed/infected bone. Although this can help reduce infection burden, it also causes bone voids where infection can thrive again and no systemic antibiotics can work effectively (Kavarthapu et al, 2023). In summary, dysfunction of several other physiological processes in a chronic wound [Figure 1]
creates a complex environment that makes it difficult to treat chronic versus acute wound
(Monika et al, 2022).
Biodegradable topical/local antibiotic delivery vehicles are emerging as a solution to assist post-surgery bone formation and provide optimal antibiotic concentration in the infected area (Markakis et al, 2018), with a number of studies on calcium sulphate beads showing improved outcomes in both infected and uninfected wounds (Tarar et al, 2021; Lun et al, 2024). Howard et al (2022) found that, for managing revision arthrodesis, layering an autologous bone graft with an adjuvant antibiotic-loaded calcium sulphate–hydroxyapatite paste can prevent infection recurrence in void bone gaps. A study of diabetes patients with transmetatarsal amputation found that bioabsorbable calcium sulphate beads with antibiotic-loading lowered the wound revision rate, suggesting that this single-stage intervention can potentially decrease hospital stay and other healthcare cost while improving patient’s quality of life (Kraus et al, 2009).
For mildly and moderately infected DFUs, topical antimicrobial treatment has been suggested as an alternative to systemic treatment and can provide a route to avoid the emergence of antibiotic resistance (Lipsky et al, 2008; Lipsky et al, 2012).
STIMULAN® – calcium sulphate beads loaded with antibiotics – releases above-therapeutic levels of antibiotics at the site of wound and has been shown to target the biofilm bioburden in people with DFIs, potentially by controlling local infection and inducing positive angiogenic effects (Crowther et al, 2021; Chadwick et al, 2022). This helps address important wound healing challenges outlined in Figure 1.
Structure and mechanism of action of STIMULAN®
Approved for use in both bone and soft tissue, STIMULAN® is a bioabsorbable calcium sulphate matrix available in different bead sizes and can be applied to versatile surgical sites (Biocomposites, 2024). The beads consist of calcium sulphate dihydrate, once prepared, and, when used for bone voids in situ, are absorbed within 30–60 days. Due to the adaptability of these beads in filling voids and gaps, STIMULAN® can be used for general surgery, cyst treatment, osteomyelitis surgery and other clinical situations involving traumatic wounds.
This clinical evaluation assessed the effectiveness of antibiotic-loaded calcium sulphate (STIMULAN®) as an adjunct therapy in the treatment of diabetic foot infections.
Patient selection and clinical evaluation design
In this single-centre clinical evaluation at King’s College London Hospital, UK, patient identification was podiatry-led, with a MDT confirming patient suitability. Once patients were selected, the choice of antibiotics was finalised according to microbiology results. Patient inclusion was based on the following criteria:
- People with diabetes of any kind who had a non-healing diabetic foot ulcer (despite receiving the best standard of care) with mild infection in the wound as per the guideline of the international Working Group on the Diabetic Foot/Infectious Diseases Society of America (IWGDF/IDSA)
- People with slow-to-heal DFUs or low-grade osteomyeltis with presumed viable bone were included as part of the evaluation. However, those with advanced bone necrosis were excluded
- No immediate revascularisation or orthopaedic surgical intervention was being considered; however, some patients may have previously received such interventions
- People who were intolerant to systemic antibiotics were also considered, as long as they also the met the above criteria
Based on this clinical evaluation design, the CCG local to the authors’ clinical setting created and approved the use of this pathway for people with complicated foot infections.
Figure 2 depicts the design and the timeline for follow-ups.
Preliminary results
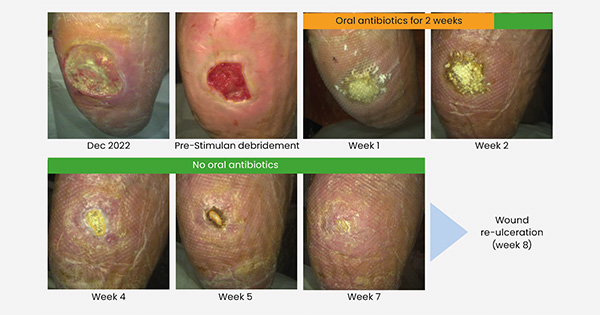
At the time of this presentation, a total of 20 people were included in the early feasibility phase, with microbiology analysis of their wounds yielding varied results. Figure 3 shows a patient’s recovery journey over the course of the clinical evaluation. This patient received no systemic antibiotics after the first two weeks, and healing of the ulcer was achieved by week 8. (Detailed outcomes and patient data will be published in a separate article.)
Adverse events
No self-reported adverse events were recorded for any patient (full data not shown here; a separate publication is in progress). Additionally, there was no systemic toxicity/adverse events and no localised, wound-site reaction to STIMULAN®. No new/flared up infection was reported either. Overall, STIMULAN® was well-tolerated in all patients.
Discussion
This initial experience indicates the impact of the MDT and safety of STIMULAN® in DFIs for people living with diabetes and soft-tissue infection or osteomyelitis. Systemic antibiotics are currently the go-to treatment for DFI management (National Institute for Health and Care Excellence [NICE), 2019). However, the effectiveness of systemic antibiotics in this patient population is compromised due to the high prevalence of PAD, dysfunctional immune, nervous and metabolic systems, and presence of necrotic tissue around the affected area; together, these abnormalities hinder the effectiveness of systemic antibiotics, decrease the probability of DFI resolution and lead to delayed wound healing in people living with diabetes (Lang et al, 2000; Turner et al, 2005).
In this backdrop and with the worldwide increase in diabetes prevalence, the need for an effective localised antibiotic treatment is rising significantly. Patient outcomes reported in this clinical evaluation are consistent with several previous reports in presenting calcium sulphate-based antibiotic delivery systems as an emerging solution (Jogia et al, 2015; Luo et al, 2016; Morley et al, 2016; Maale et al, 2020).
Local antibiotic delivery can potentially lead to some adverse events similar to systemic antibiotic treatment (e.g. allergy). In vancomycin case studies with cobalt-cement and cobalt spacers, antibiotic toxicity has been reported (Reed et al, 2014; James and Larson, 2015). However, antibiotic-loaded calcium sulphate (STIMULAN®) was well-tolerated in the patient cases presented here. No patients reported any discomfort or any other problem with their treatment. There were no systemic adverse events, local reaction to STIMULAN® or unanticipated new/flared-up infections. This is consistent with previous STIMULAN® studies where no adverse effects were recorded (Chadwick et al, 2022). One exception in these studies was the presence of skin maceration which could be attributed to the effect of bead dissolution. However, this adverse event did not occur in the population included in this clinical evaluation. On the other hand, systemic antibiotic treatment for diabetic wound infections can result in several different adverse events including allergy, Clostridium difficile infection, adverse impact on liver and kidney function and emergence of antibiotic-resistant bacteria (Tamma et al, 2017).
Local antibiotic treatments can help avoid some of the systemic adverse outcomes reported with systemic antibiotic treatments (e.g. organ toxicity) without causing any significant additional adverse events (Crowther et al, 2021). Furthermore, the ease of use and reduced need for hospital stay can also help decrease overall clinician time and healthcare costs currently associated with unhealed or complex diabetic wounds (Morley et al, 2016; Chadwick et al, 2022).
Not only do these data indicate improved outcomes for people currently included in this clinical evaluation, this treatment pathway can be adapted as per local guidelines by CCGs in other clinical settings across the UK for people living with complicated DFIs.
The team are currently preparing a manuscript, based on the 12-week outcome of a larger-scale clinical evaluation and, therefore, further data is awaited. The current clinical evaluation demonstrates that calcium sulphate-based, localised antibiotic carriers can provide a safe alternative or adjunct to systemic antibiotics while avoiding the adverse outcomes associated with systemic antibiotic treatment of DFIs.
Conclusion
STIMULAN®, a pharmaceutical-grade calcium sulphate antibiotic carrier, offers a well-tolerated option for improved healing in nonhealing and/or complex diabetic foot wounds. Created as beads that can fill ‘dead spaces’, this system has the capacity to deliver an effective local concentration of an antibiotic, a goal that is hard to achieve with systemic antibiotic therapy in people living with diabetic foot ulcers. When using this intervention, the initial patient management should be undertaken in a formal multidisciplinary setting and there is a need to identify patient groups that can receive maximum benefits from this intervention. Finally, prospective randomised clinical trials are needed to fully assess the effectiveness of this intervention and monitor for emergence of resistant bacterial strains.