Surgical site complications include surgical site infections (SSI), wound dehiscence, seroma or haematoma, which lead to delayed or impaired healing (Scalise et al, 2015). For some laparotomy surgeries, the incidence of SSI has been found to be as high as 37% (Maehara et al, 2017). Individuals with risk factors, such as obesity, type 2 diabetes, chronic kidney disease, >75 years, immunosuppression and smoking, are more likely to experience surgical site complications (World Union of Wound Healing Societies [WUWHS], 2016).
Minimising surgical site complications can lead to reduced healthcare costs and improved patient outcomes, including reduced morbidity, reduced psychological effects for patients, shorter hospital stays and avoidance of readmission to hospital (WUWHS, 2016). Treatment that reduces the risk of complications must be explored and used for improved patient safety and associated impacts.
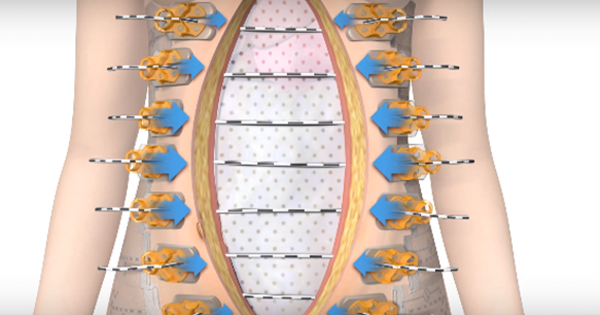
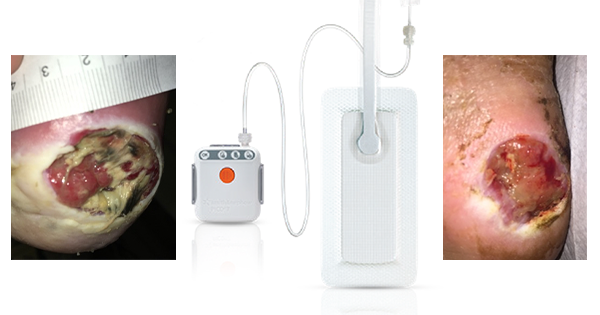
Negative pressure wound therapy (NPWT) has been used for the treatment of chronic wounds and complex surgical wounds for more than 20 years. Single-use NPWT is now used prophylactically for patients with closed surgical incisions (National Institute for Health and Care Excellence [NICE], 2019). The risk of dehiscence is reportedly reduced by decreasing lateral tension along the incision. Additionally, other benefits to wound healing have been reported, including reduced SSI, increased blood flow and lymph filtration; leading to a reduction in seroma or haematoma formation (Saunders et al, 2021). The cost of single use NPWT in closed surgical incisions has been found to save approximately £105 per patient (Nherera et al, 2021).
Methods
A literature review was undertaken to identify current research. Search terms included “single use NPWT”, “laparotomy wounds” and “PICO”. An initial search located 32 papers on the use of single use NPWT for laparotomies. Exclusion criteria included case series and case control studies to ensure an appropriate hierarchy of evidence was used. The search was limited to papers published in the last 4 years to ensure the most recent evidence base was reviewed.
Results
Three studies [Table 1] and two reviews [Table 2] were suitable for inclusion.
An RCT by Andrianello et al (2021) compared the use of single use NPWT with a standard post-operative dressing in high-risk patients following pancreatic resections, with demographics stratified between the two groups. There was no difference in superficial or deep SSI (p=0.709 and p=0.618 respectively) or the prevalence of haematoma (p=0.609) between the two groups. However, statistically significant results showed that participants in the intervention group did not have any seromas reported, compared to the control group incidence of 12.2% (p=0.027).
Similar results were discussed in a retrospective comparative study comparing the prevalence of SSI in patients following elective loop ostomy closure (Curchod et al, 2022). Single use NPWT (n=85) was used for 5 days post operatively and was compared to a control group using surgical glue as the wound dressing (n=252). Results showed that there was no statistically significant difference in SSI (p=0.097), length of stay (p=0.058) or return to theatre (p=0.981) between the two groups.
A limitation of this retrospective study is the reliance on accurate documentation of outcomes. It has been recognised that capturing clinical outcomes is undervalued by some clinicians, which can lead to inaccuracy (Elkbuli et al, 2018). The study design used by Curchod et al (2022) is at risk of observational bias (Aveyard, 2018). Furthermore, the retrospective sampling utilised in this study did not allow for random sampling; thus increasing the likelihood of selection bias (Aveyard, 2018). These factors impact upon the validity of the study. Whilst the researchers introduced single use NPWT based on these results in their area, when viewed in isolation, the evidence does not provide robust findings to implement a change in practice.
Andrianello et al (2021) acknowledge that although the dressings in both the intervention and control group were supplied by the manufacturer, the company was not involved in the analysis of results; thus, the reduced risk of bias should be acknowledged. While Curchod et al (2022) used the same manufacturer, there was no information as to whether the study was funded by the company or whether they participated in analysis of evidence; potentially increasing the risk of bias. Therefore, the results of that study should be viewed with caution, particularly as the researchers decided to implement single use NPWT in their practice.
An RCT by Wierdak et al (2021) investigated the use of single use NPWT (n=35) compared to conventional dressings (n=36) following ileostomy reversal to prevent wound healing complications in colorectal patients. SSI prevalence was found to be lower in the intervention group compared to the control group (5.71% versus 22.2%, p=0.046). Furthermore, wound healing complications were also lower in the intervention group compared to the control group (8.57% compared to 30.6%, p=0.02). A significant limitation of this study was that only 65% power was achieved, rather than the desired 80%, meaning the results were underpowered (i.e. the sample size was not large enough to produce reliable results). Therefore, the results should not be considered to be statistically significant due to a type II error (Shreffler and Huecker, 2022). This study should be viewed in combination with other studies with adequate sample sizes that have statistically significant results when deciding whether to implement single use NPWT in practice.
A Cochrane review by Webster et al (2019) assessed the effects of NPWT for preventing SSI of wounds healing through primary closure through examining 30 RCTs that included a total of 2,957 participants. However, this review looked at all types of NPWT rather than solely single-use NPWT. Each study was reviewed and the bias risk evaluated. Following a systematic review of all evidence, it was found that NPWT may reduce the rate of SSI compared to conventional dressings; however, this evidence was low quality. Evidence for NPWT reducing the rate of dehiscence, seroma or mortality was also of poor quality (Webster et al, 2019).
The review highlighted that evidence was of low quality due to the high risk of bias and many of the RCTs conducted had small sample sizes. Both Andrianello et al (2021) and Curchod et al (2022) acknowledged that their studies had low sample sizes due to their exclusion criteria, which impacts on the internal and external validity. Larger sample sizes would improve confidence limits that the null hypothesis can be accepted or rejected (Aveyard, 2018). Both studies included patients at high risk of SSI, thus improving the generalisability of both studies.
More recently, a Cochrane review by Norman et al (2022) compared conventional wound dressings and NPWT for surgical wounds healing by primary closure. Despite the review including 62 RCTs and 13,340 participants, the authors found that prophylactic use of NPWT does not affect frequency of wound dehiscence (6.62% incidence) compared with conventional dressings (6.97% incidence), nor is one treatment is more cost effective than the other.
However, the review reported that the frequency of SSI (n=11,403) is likely to be lower in NPWT (8.7% of participants) than conventional dressings (11.75% of participants). This an important finding, as each SSI is thought to cost an average of £5,239 (Jenks et al. 2014). Norman et al (2022) concluded that the prophylactic use of single use NPWT can improve patient experience outcomes, while reducing associated costs of SSI for the healthcare provider.
A limitation of both Cochrane reviews when examining whether to introduce single use NPWT for laparotomy wounds, is the inclusion of all types of surgery, including abdominal, vascular, arthroplasty and spinal surgery. Additionally, both reviews included studies using single-use NPWT and conventional NPWT devices. This will influence the transferability of the evidence when considering the introduction of specifically single-use NPWT in laparotomy wounds. These reviews should be considered alongside evidence which specifically uses single-use NPWT when choosing to implement a change in practice. Despite this, these reviews provide valuable evidence in using NPWT prophylactically to decrease the frequency of SSI, particularly in the more recent review by Norman et al (2022).
The NICE guidance (2019) supports the use of PICO, a brand of single-use NPWT, for closed surgical incisional wounds. The guidance reviewed 15 RCTs and 16 non-randomised observational studies (n=1,804) and found that on collation of all evidence, there was a statistically significant reduction in SSIs when using PICO (p=0.006).
Similar to the Cochrane reviews, NICE (2019) collated evidence from six types of surgery to assess the impact of NPWT with 1 RCT and 10 observational studies for colorectal surgery. This will affect the generalisability of the results when looking to introduce single use NPWT in laparotomy wounds and therefore should be considered alongside other studies.
Wilkin et al (2021) have published a proposed trial protocol which aims to explore whether single use NPWT decreases the risk of SSI following emergency laparotomy compared to conventional dressings using a large sample size (n=840). The proposed trial will be a RCT with outcome assessors blinded to treatment allocation, thus decreasing observer bias. Once published, this trial may provide statistically significant evidence to support the use of single use NPWT due to the robust study methods employed.
Discussion
Exploration and analysis of available evidence demonstrates there is currently little high-quality research and evidence to support the use of single use NPWT in preventing surgical site complications for laparotomy wounds. However, new emerging research shows that there is a positive benefit of single use NPWT to reduce SSIs, although many of the studies highlight the need for further high quality RCTs.
Some limitations of this paper should be acknowledged. This is a critical analysis completed by a lone author. Although the depth is not that of a full literature review, it does reflect the realities of evidence synthesis and consideration of changes in practice for clinicians working in the field of tissue viability.
Implications for practice
The strength of evidence is such that locally, only a small-scale change is proposed. The evidence is insufficient to implement a high-cost trust wide change in practice and it is clear that further high-quality research is required.
Conclusion
The appraisal of evidence to date suggests that single-use NPWT may reduce SSI and wound site complications in patients undergoing laparotomy. New evidence should be routinely reviewed and analysed as emerging research is available. This will ensure evidence is of high quality with sufficient sample sizes to demonstrate whether there is a statistically significant difference in surgical wound complications when using single-use NPWT.