Sickle cell leg ulcers usually occur around the ankle and have very poor healing rates, with a detrimental impact on quality of life, particularly as they usually develop in patients who are in their 20s (Marti-Carvajal et al, 2021).
Most of the literature in this area is international, because the prevalence of sickle cell disease (SCD) is higher in people of sub-Saharan African, Indian and Middle Eastern origin (Marti-Carvajal et al, 2021). The incidence of ulceration in SCD varies hugely across the globe (Madu et al, 2022). Rates of leg ulcers in patients with SCD vary from 75% in Jamaica to 1% in Saudi Arabia and the US (Minnitti et al, 2016; Sahu et al, 2021). The causes for the significant international variation in prevalence of ulceration in patients with SCD appears to be multifaceted and is an interesting area which requires further study. It is likely to be related to different genotypes, methods of measurement and definition, as well as environmental factors (Madu et al, 2022). The differing theories on the pathophysiology of sickle cell leg ulcers will be included in this article.
The treatment options to be examined are:
- Nutrition optimisation, focusing on zinc
- Topical sodium nitrite
- Standard treatments for wound healing, such as wound bed preparation and compression bandaging
- Hydroxyurea, and voxelotor, drug therapies in SCD.
These treatments are discussed in the context of a tissue viability service in a large tertiary centre caring for patients with SCD with leg ulcers who present in the SCD clinic.
The effect of SCD on wound healing
The normal physiology of wound healing is impacted by SCD (Minniti et al, 2014a). SCD causes red blood cells to be misshapen; people with SCD have an abnormality of haemoglobin S (Midence and Ellander, 1994). The haemoglobin molecule is made of two alpha and two beta globin chains and the haem molecule; the SCD mutation is in the beta chain (Sickle Cell Society, 2018).
SCD will be used to cover the range of sickle cell disorders, although the prevalence of leg ulcers is more common in the HbSS genotype (Anionwu and Atkin, 2001). HbSS occurs where the beta globin gene is inherited from both parents and those who have this genotype tend to experience the most severe complications (Marti-Carvajal et al, 2021). When deoxygenised, the molecules of haemoglobin S can come together, causing the haemoglobin to thicken and stiffen, which leads to the sickled shape. The red blood cells have very rapid haemolysis, in comparison to healthy red blood cells (Midence and Ellander, 1994).
The misshapen cells lead to blockages in the smaller blood vessels. This vaso-occlusion and the rapid haemolysis contribute to inflammation of the vasculature and endothelial damage, which can lead to end organ damage, stroke, pulmonary hypertension, renal damage and reduced life expectancy (Anionwu and Atkin, 2001).
People with SCD experience vaso-occlusive crises, and these can be incredibly painful, at times requiring hospitalisation and analgesia, often opioids (Midence and Ellander, 1994; Carulla et al, 2023). The rapid haemolysis, which is death of the red blood cell, can cause anaemia (Anionwu and Atkin, 2001).
Understanding the aetiology of a wound is vital to effective treatment (Vuolo, 2009). Normal wound healing involves a complex cascade of overlapping processes, which are haemostasis, inflammation, proliferation and maturation. Wounds can become stuck in the inflammation phase due to bacterial burden and the presence of necrotic tissue, which can be managed by wound bed preparation (Atkin et al, 2019). Ulcers in people with SCD often do not progress through the stages of healing and may well become stuck in the inflammatory phase. When reviewing leg ulcers in people with SCD using laser speckle contrast imaging, Minniti et al. (2014a) found high levels of inflammation, despite all participants having leg ulcers that were present for more than 4 weeks.
Patients with SCD can often have low haemoglobin and foetal haemoglobin, which is argued by some authors to lead to leg ulceration (Montford and Senet, 2020). Foetal haemoglobin is the form of haemoglobin present at birth which the body gradually replaces with adult haemoglobin. Foetal haemoglobin is protective against sickling (Sickle Cell Society, 2018).
SCD leg ulcers usually occur around the malleolus, an area that has reduced blood flow (Minniti et al, 2021). Anaemia and haemolysis damage the vasculature, and lead to end organ hypoxia, which may cause leg ulceration as well as other complications of SCD (Vichinsky et al, 2019). End organ hypoxia is caused by vasoconstriction and occlusion of the vasculature (Sickle Cell Society, 2018). Severe anaemia characterises most end organ damage for patients with SCD disease.
Rapid haemolysis leads to a low availability of nitric oxide, which is also cited as a cause of poor wound healing (Montford and Senet, 2020). Reduced nitric oxide can lessen the functioning of the endothelium because nitric oxide regulates homeostasis in the vasculature and vasodilation (Marti-Carvajal et al, 2021 and Minniti et al, 2016). In SCD, the endothelium is impacted by adhesion of blood cells to the vessel lining, damaging the vasculature and leading to blockages and painful sickle cell crises (Sickle Cell Society, 2018).
Other authors cite the impact of poor perfusion, thrombosis and damage to the microcirculation (Sahu et al, 2021). This is thought to lead to ischaemia, which in conjunction with susceptibility to infection leads to poor healing and high recurrence rates of leg ulcers in the SCD population (Marti-Carvajal et al, 2021). There is also thought to be the risk of reperfusion injury leading to tissue damage caused by the ischaemia and subsequent perfusion (Young, 2020).
Treatment options for SCD
Medications
A Cochrane review examined six studies, on systemic and topical treatments for SCD (Marti-Carvajal et al, 2021). The systemic treatments were L-cartinine, isoxsuprine and arginine butyrate, of which the latter showed the most promising results with a reduction in ulcer size of the control group and 8% healing rate in ulcers after 12 weeks (McMahon, 2010). However, the administration of arginine butyrate is 12 weeks of IV therapy and this poses challenges for administration, as well as potential adherence issues (Marti-Carvajal et al, 2021).
The topical treatments included a triple topical antibiotic (Marti-Carvajal et al, 2021). Topical antibiotics are usually avoided in wound healing due to the risk of antimicrobial resistance, and topical antiseptics are the first option in most cases (National Institute for Health and Care Excellence [NICE], 2014; Edmiston et al, 2017).
In their literature review on leg ulcers in SCD, Sahu et al. (2021) highlighted positive outcomes in bone marrow mononuclear stem cell therapy. Currently, bone marrow or stem cell transplantation are the only curative treatments for SCD (Connor et al, 2017). Connor (2017) describes a case of a patient with SCD with an active leg ulcer had a stem cell transplant, which was effective in treating SCD. Of note, an open wound can be a barrier to receiving a stem cell transplant, regardless of how the leg ulcer healed post-transplant. The patient in this case had experienced leg ulceration for over 20 years. Although this is just one patient, this result is encouraging.
In wound care, obtaining high quality evidence is challenging due to the complexities of co-morbidities and the changing nature of the wound bed (World Union of Wound Healing Societies [WUWHS], 2020). A further challenge within the SCD leg ulcer patient group is its small size, and this has led to a limited availability of high-quality evidence for treatments in this area. Therefore, other forms of evidence need to be utilised in wound care, as emphasised by the European Wound Management Association (EWMA) in their paper on outcomes in controlled and comparative studies on non-healing wounds (EWMA, 2010).
Voxelotor
In May 2024, NICE recommended the use of voxelotor for patients with SCD (NICE, 2024). Voxelotor has been shown to inhibit polymerisation and reduce haemolysis (Minnitti et al, 2021).
A recent randomised placebo-controlled trial examined the safety and efficacy of voxelotor administered at two doses, 1500mg and 900mg (Vichinsky et al, 2019). The aim was to measure the impact on haemoglobin, and the authors concluded that voxelotor does lead to an increase in haemoglobin, and for most this occurred within 2 weeks and continued throughout the monitoring period.
In this trial, which ran over 72 weeks, 13 of the 274 enrolled patients had an active leg ulcer, 33 reported a history of leg ulcers, and 9 patients developed a leg ulcer during the trial period (Minnitti et al, 2021). More than 70% of the patients on voxelotor had their leg ulcers heal by week 24, and these were associated with an increased haemoglobin and reduced haemolysis. The numbers of patients included with leg ulcers were small, but this updated NICE guidance on the availability of voxelotor is very encouraging for patients in England.
Hydroxyurea
Hydroxyurea is a common drug therapy for SCD, but there is no consensus as to its impact on wound healing (Sahu et al, 2021). It is thought to reduce complications of SCD by raising foetal haemoglobin and inhibiting sickling (Rankine-Mullings and Nevett, 2022). In a retrospective study by Tolu et al. (2022) on the impact of hydroxyurea and foetal haemoglobin on leg ulcers in SCD, hydroxyurea does not appear to impair wound healing. However, hydroxyurea has been reported to cause damage to the epidermis and the microcirculation (Sahu et al, 2021).
In a Brazilian study, Lopes et al (2014) found in vitro and in vivo that hydroxyurea had anti-angiogenic effects in retinopathy. Angiogenesis in wound healing in SCD may become unbalanced due to disruption of growth factors and a hypoxic wound bed (Antwi-Boasiako et al, 2018). Therefore, the variation in reported impact of hydroxyurea on wound healing could be due to the balance of pro and anti-angiogenic factors present in the wound bed prior to commencement of hydroxyurea therapy.
Foetal haemoglobin is thought to be the key in the development of SCD complications, and ulceration can be seen as another complication of SCD. In a retrospective study Tolu et al (2022) aimed to establish the range of foetal haemoglobin required to prevent leg ulcers developing, and the impact of hydroxyurea on existing leg ulcers. Their study included 769 patients, 499 of which were taking hydroxyurea, and 146 had a history of, or active leg ulcers. The authors found that foetal haemoglobin was only likely to be protective against ulceration at a level of 25.3%. This level is uncommon, so further analysis is required as to why hydroxyurea use is not achieving this, whether due to adherence, side-effects or the drug itself. This requires further examination within the wider multidisciplinary team caring for patients with SCD, potentially with those patients attending in the clinic setting.
Oral sinc
A Cochrane review examining the impact of oral zinc on arterial and venous leg ulcers found there was no significant impact on wound healing (Wilkinson, 2014). The review included six small trials, four on venous leg ulcers, one on mixed aetiology leg ulcers and one on arterial leg ulcers.
However, in a review of treatments in SCD leg ulcers Montford and Senet (2020) reported a small single randomised control trial from 1970 demonstrated optimising zinc levels, typically low in patients with SCD, could improve the speed of wound healing (Sergeant et al, 1970). But this trial only included 15 in the control group and 14 in the placebo arm. No further research into zinc levels in SCD wound healing appears to have been performed since 1970.
Optimising the nutrition of SCD patients seen in clinic, working with the nutrition team, and monitoring levels of zinc alongside wound healing would be practical within the clinic setting in our tertiary centre. The Sickle Cell Society published standards of care that included monitoring of zinc levels and use of supplements for those with leg ulcers (Sickle Cell Society, 2018).
Topical sodium nitrite
Nitric oxide (NO) is vital to many aspects of wound healing as it supports the production of the extra cellular matrix, keratinocyte proliferation and angiogenesis (Montford and Senet, 2020). Haemolysis releases haemoglobin, which then consumes NO (Marti-Carvajal et al, 2021). Topical sodium nitrite is known to aid in generating nitric oxide and to support vasodilation (Minniti and Kato, 2016).
In a Phase I single centre trial, Minniti et al. (2014b) looked at the dosage required for safe and effective use of sodium nitrite. This study included 18 participants with ulcers of more than 4 weeks’ duration, with a size of 2.5–100cm2. The study demonstrated topical sodium nitrite was safe and wound healing was improved, particularly in the group receiving the higher dose of 2% sodium nitrite, where wound size decreased by 88% and three ulcers healed in the participants overall. Wound healing was measured using visible light photographs taken at a set distance from the wound, with software calculating the surface area. This method aimed to make the assessment as objective as possible. However, this technology is not available to most clinicians, which can potentially lead to variation in care provision and results. The study size was small, and so further research is required, but the results are promising for an adjunct treatment for leg ulcers in SCD patients.
In a qualitative prospective study, Connor et al. (2018) demonstrated a positive impact of sodium nitrite on quality of life. The study collected surveys from the participants in the sodium nitrite phase one trial above (Minniti et al, 2014b) and a control group. These examined the impact of leg ulcers on aspects of patients’ everyday life, including work, sleep, mental health, family relationships and the burden of leg ulcer care. The authors used a Medical Outcome Study Short Form Survey and participants reported better physical outcomes, such as less pain and improved social functioning, and improved mental wellbeing in those age 35 and under after the trial of topical sodium nitrite.
These preliminary and small-scale studies show topical sodium nitrite could have potentially wide-ranging positive impacts on patients with sickle cell leg ulcers. Topical sodium nitrite is not currently licensed in the NHS, so further research is needed to demonstrate its safety and efficacy, and this could lead to a change of clinical practice (NHS, 2020).
Standard treatments
Minniti et al. (2014a) used laser speckle contrast imaging to examine the blood flow to the ulcer site and compared it to an ulcer free area of the same limb. The ulcers had increased blood flow, in comparison to the ulcer free area. The authors posit the increased blood flow to the wound and peri-wound could indicate the ulcers were stuck in the inflammatory phase. The ulcers were present for a median duration of 10 months.
However, these results could challenge the view held by many that poor perfusion causes ulcers in SCD (Marti-Carvajal et al, 2021). Many hard-to-heal wounds become stuck in the inflammation phase, due to biofilm and cell senescence, and there is evidence to support means of getting these wounds out of stasis (Atkin et al, 2019). This includes sharp/mechanical debridement and dressings that are anti-inflammatory/protease modulators, which has evidence of efficacy in diabetic foot ulcers (Bianchi, 2001; Richard et al, 2012). These are treatments which are available in practice now. However, anecdotally, even with analgesia, sharp debridement is not always possible due to the significant pain associated with the ulcers. Treatment of leg ulcers for patients with SCD often includes the recommended treatment for venous leg ulcers, compression bandaging (Senet et al, 2017).
It has been reported that SCD leg ulcers have healing rates of 16 times slower than venous leg ulcers (Connor et al, 2014). In a 2017 prospective observational cohort study, Senet et al. found that the size of the leg ulcer and duration of the leg ulcer had a greater impact on healing at 24 weeks than characteristics associated with SCD, such as haemolysis or end organ damage.
From these results , a treatment plan based on holistic assessment, wound bed preparation and compression (where appropriate and tolerated) can be developed. But the question of why there are poorer healing rates within the SCD population compared to venous leg ulcers remains (Connor et al, 2018). EWMA (2010) recommended the time to wound closure measured should be 12 weeks, but that this would not be appropriate for every wound type. The poor healing rates in SCD might mean longer time to healing is useful to examine, but if that is done, the standardisation of 12 weeks is lost, impacting the ability to compare studies and treatments effectively. In practice it may be more realistic to discuss with the patient and team that leg ulcers in patients with SCD may have a longer trajectory of healing.
Senet et al. (2017) categorised a leg ulcer as a non-healing ulcer present for ≥2 weeks, differing from other studies. In the UK the National Wound Care Strategy Programme (NWCSP) recommends a wound to the lower limb be defined as a leg ulcer if it is present for >2 weeks (NWCSP, 2023). As reported by Minniti and Kato (2016), many studies also do not differentiate between an active ulcer and a history of leg ulcers. Without standardised definitions and language, it is not possible to compare evidence and compile meta-analyses (WUWHS, 2020).
In a recent case study of a holistic treatment algorithm for SCD leg ulcers, Rivolo (2018) reported wound healing in 6 months, where the patient’s ulcer had been present for 4 years. The method used a combined approach of infection control, wound bed preparation, exercise, compression and emotional support. It is difficult to be able to show causation with a case study, however Rivolo reports success with wound healing in 6 months with a further five patients using this approach. There is evidence to show in other non-healing wounds the benefit of these therapies (NWCSP, 2023). This area could be a basis for further research.
Case study
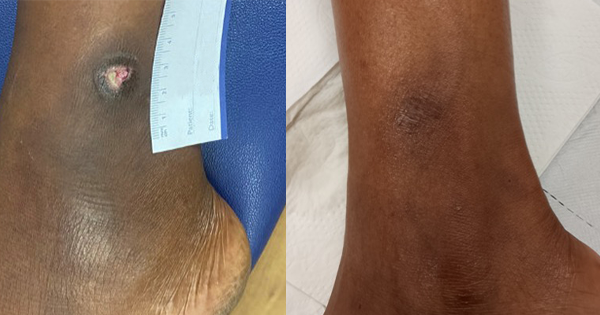
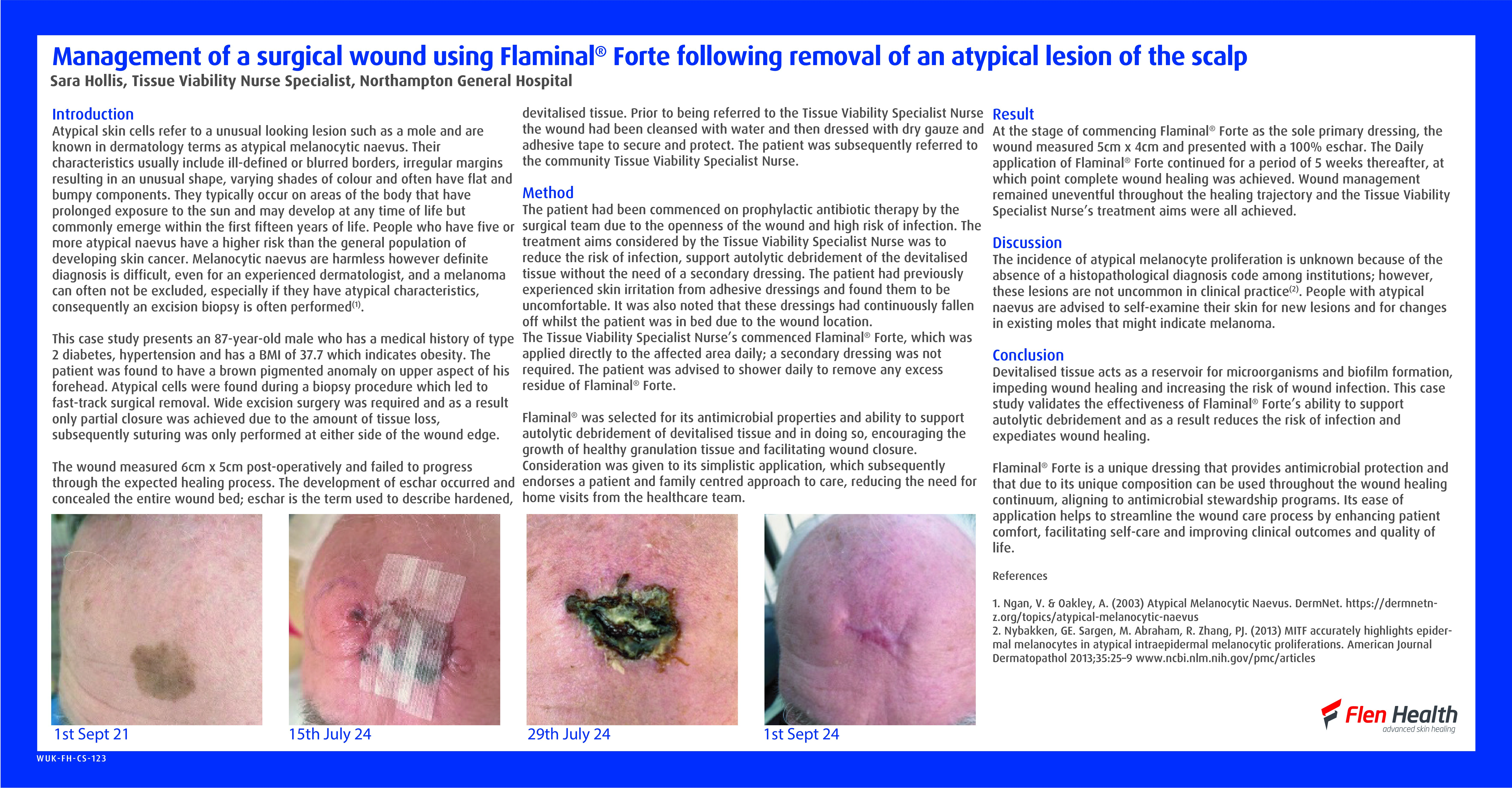
The Tissue Viability Team were asked to see a patient in her early 30s will HbSS sickle cell anaemia. The patient was primagravida, in her second trimester, and had developed an extremely painful leg ulcer on her left lateral malleolus. The wound was less than 1cm by 1cm, with a shallow wound bed covered in slough, with moderated exudate [Figure 1].
An ABPI was completed, and the patient was deemed to be safe for full compression. Unfortunately, this was not started immediately as she was out of area and had problems accessing primary care.
The patient was working full time in a role that involved a lot of time on her feet.
As the pregnancy developed, so too did the leg ulcer [Figure 2]. Several topical treatments were attempted, but many were too painful for the patient to tolerate, as was compression once the stockings arrived.
The patient was understandably cautious about increasing her analgesia due to her pregnancy. The pregnancy also meant she was not able to use other medications for her SCD, such as hydroxyurea. It also presented increased complications for blood transfusion.
There was no possibility of sharp/mechanical debridement, even cleansing the wound bed caused the patient incredible pain. The wound remained shallow, but surface area increased in this time.
At around 35 weeks pregnant, the patient was admitted with a sickle cell crisis, and was give morphine via patient-controlled analgesia. However, she was weaned off morphine over a few days due to reduced foetal movement. The pregnancy was also causing jaundice and increased haemolysis. The patient was admitted for under 2 weeks, and the enforced bed rest was the first time a reduction in the size of the ulcer was seen [Figure 3]. The patient also received two blood transfusions in this time.
After discharge, the patient was signed off work and the ulcer continued to reduce in size with leg elevation, the use of full compression stockings and hydration of the wound bed with a hydrogel, which the patient was able to self-manage at home. The patient was totally healed 6 months later [Figure 4].
I can happily report mother and baby are both doing well.
Discussion
This case study highlights some important aspects for clinicians encountering patients with SCD and a leg ulcer. Pain to the ulcer site can mean compression therapy and sharp/mechanical debridement are not always an option in SCD. In this case study, bed rest led to the greatest improvement in the leg ulcer. However, as SCD leg ulcers typically present at a young age, bed rest poses a significant challenge to the patient with regard to education, family and working life (Anionwu and Atkin, 2001).
Conclusion
There is a lack of clarity about the pathophysiology of sickle cell leg ulcers. The available evidence is difficult to obtain, mainly due to small numbers, and the lack of uniformity in definitions. But the evidence reviewed in this article does lead to some interesting conclusions, notably, challenging the view that hydroxyurea is damaging to wound healing, and this can support its continued use in patients with severe disease to protect against end organ damage.
Voxelotor is now available for NHS patients, a very promising advance. Viewing SCD as a risk factor for leg ulcer development, rather than leg ulcers in SCD being of a unique pathophysiology, suggests the use of wound bed preparation and compression bandaging in this group. These treatments are the current practice for SCD leg ulcers locally.
Further investigation of the impact of topical sodium nitrite would be beneficial; the results from the early trial are promising. A holistic approach is required to target both the wound and the wider systemic effects of SCD, such as pain control and optimising zinc levels.
Finally, the impact of the different genotypes and geographical locations of leg ulcer prevalence appear to be important; it would be beneficial to conduct further research in this area.