This article is based on a Made Easy workshop held at the Wounds UK Annual Conference in Harrogate, UK, on 6th November 2023. The workshop and this report were sponsored by Flen Health.
Steven Percival opened the workshop by introducing antimicrobial resistance (AMR) and methods to reduce AMR, and Leanne Atkin went on to introduce Flaminal®, an enzyme alginogel, and gave guidance on managing wounds in difficult-to-dress areas.
Antimicrobials
Antimicrobials, including biocides, antibiotics, antiseptics, disinfectants, antivirals, antifungals and antiparasitics, are products or medicines used to prevent and treat infections in humans, animals and plants (World Health Organization [WHO], 2021).
Biocides
Biocides, such as QAC (algaecide), function to destroy, deter, render harmless or exert a controlling effect on any harmful organism through chemical or biological means.
Antibiotics
Antibiotics, such as penicillin, are the only antimicrobial compounds that can also be administered systemically. They exhibit selectivity by targeting specific sites within bacterial cells, proving toxic and effective in destroying microbes within the body. However, the widespread use of topically and systemically administered antibiotics often leads to the development of resistance (Nair et al, 2023).
Antiseptics
Antiseptics, such as alcohol and silver, are topical antimicrobial agents with broad-spectrum activity that inhibit the growth and multiplication of microorganisms and can also kill them. The impact on human cells is concentration-dependent, with higher concentrations potentially exhibiting toxic effects. However, modern antiseptics generally demonstrate good tissue compatibility, and the development of resistance to topical antiseptics is considered less common compared to antibiotic resistance (Haesler et al, 2022).
Disinfectants
Disinfectants, such as chlorine, polyhexamethylene biguanide and peracetic acid, are substances that exhibit non-selective antimicrobial activity and are recommended by the manufacturer for application to non-living objects to kill microorganisms such as bacteria, viruses and fungi (IWII, 2022).
Introducing antimicrobial resistance (AMR)
AMR occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines making infections progressively more difficult or even impossible to treat (WHO, 2021).
AMR is a growing public health challenge worldwide and is recognised as one of the top 10 threats to global health (WHO, 2021). It is estimated to be responsible for at least 10 million deaths annually and will cost approximately $100–200 trillion USD globally by 2050 (Interagency Coordinating Group on Antimicrobial Resistance, 2019).
Causes of AMR include:
- Misuse and overuse of antimicrobials
- Poor infection and disease prevention and control in healthcare facilities and farms
- Limited access to quality, affordable medicines, vaccines and diagnostics
- Limited education, awareness, and understanding that not all conditions necessitate the use of antimicrobials
- Inadequate access to clean water, sanitation and hygiene
- Lack of enforcement of legislation.
Why is AMR a concern?
Resistant microorganisms can potentially lead to the spread of resistance in both the community and healthcare facilities — e.g. a patient with AMR can transmit resistant microorganisms to other patients through poor hygiene practices, close proximity and contaminated objects, such as unchanged hospital bed sheets shared among different patients. See Box 1 for additional consequences of AMR.
Mechanisms of resistance development
Microorganisms can possess intrinsic or innate resistance properties embedded in their DNA, or they can acquire resistance through mutations. Additionally, resistance can be developed through the acquisition of plasmids (self-replicating, extrachromosomal DNA) or transposons (plasmid integrating or chromosomal, transmissible DNA cassettes), which typically occur in a biofilm (McDonnell and Russell, 1999).
Biofilm
Biofilms are complex polymicrobial communities, attached either to each other or to a biotic or abiotic surface (not necessarily a solid surface), and become encased within an extracellular polymeric substance. They can increase the likelihood of a wound becoming infected and prevent wound closure (Wolcott et al, 2016; Kadam et al, 2019).
Biofilms can’t be seen with the naked eye; however, the consequences of unmanaged biofilms can look like:
- Delayed wound healing
- Visible, slimy, gel-like shiny cover (slough) on the wound bed, which detaches easily and can be peeled off the wound without trauma to the wound bed
- Rapid reforming of slough/slimy covering
- Increased exudate
- Poor/fragile granulation tissue
- Signs of local infection
- Persistent recurring infection
- Slow or no response to antimicrobial dressings
- Low-level chronic inflammation
- Low-level erythema.
Persister cells and biofilm
Persister cells, found within biofilms, exhibit resistance to antibiotics and are implicated in the persistence of chronic infections. While antimicrobials kill the majority of cells, persister cells remain viable even in the presence of antimicrobials and contribute to the repopulation of biofilms when the concentration of antimicrobials decreases (Lewis, 2010; Wood, 2013).
Methods to reduce AMR:
- Increase the ability to recognise infections and prevent them in the first place
- Improve antibiotic and antifungal use to reduce the risk of resistance development
- Prevent, diagnose and manage biofilms more effectively, while keeping in mind that not all biofilms are harmful
- Stop the spread of resistance once it develops
- Increase the knowledge of systemic infection options and understanding the difference between local and systemic infections
- Collaborate with the wider multidisciplinary team – e.g. microbiology, diabetology, and pharmacy, to help with complex cases
- Stay informed about local policies and specific antibiotic algorithms.
Wound assessment
As part of the immediate care for leg ulcers, the first step involves wound bed preparation (National Wound Care Strategy Programme, 2023). It is recommended to use frameworks that offer a systematic approach to wound bed assessment – such as the T.I.M.E. principle – Tissue, Infection, Moisture and Edges of the wound (Schultz et al, 2003).
Flaminal®
Flaminal® is a primary wound dressing, categorised as an enzyme alginogel. Its unique composition and modes of action make Flaminal® suitable for addressing all aspects of T.I.M.E. [Table 1], eliminating the need for multiple products. It can be used throughout all stages in the wound infection continuum (International Wound Infection Institute, 2022) and offers continuous wound debridement, broad-spectrum antibacterial activity without cytotoxicity [Box 2], absorption of excess exudate, maintenance of a moist healing environment, and protection to the wound edges and epithelial cells (Beele et al, 2012).
Mechanisms of action
Flaminal® is composed of a hydrated alginate matrix alongside its unique antimicrobial GLG enzyme system — glucose oxidase combined with lactoperoxidase stabilised by guaiacol (De Smet, 2009; Cooper, 2013). These two naturally occurring enzymes — lactoperoxidase found in breast milk and secretions of exocrine glands such as saliva, tears and cervical mucus — work together with glucose oxidase to form free oxygen radicals via hydrogen peroxide. This process is similar to the action of our innate white cell defences, leading to the destruction of the cell walls of absorbed bacteria (White, 2006).
Flaminal® is available in two formulations:
- Flaminal Hydro®: for slightly to moderately exuding wounds
- Flaminal Forte®: for moderate to highly exuding wounds.
Managing difficult-to-dress wounds
Flaminal® is versatile and can be applied to various wound aetiologies, types and conditions — see Box 3 for examples.
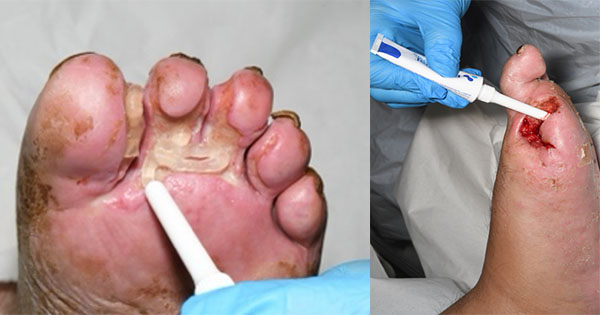
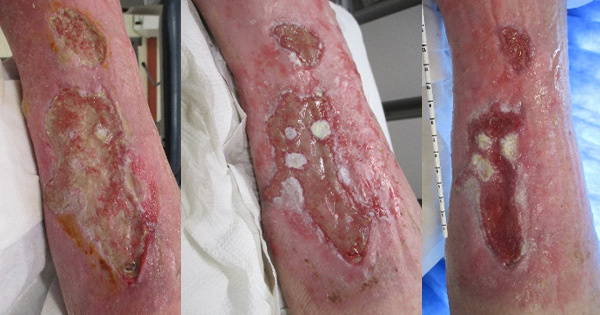
Application methods include direct application from the tube in shallow wounds, onto secondary dressings in larger wounds – e.g. leg ulcers, with a syringe or nozzle for deep cavity wounds, wound edges or toes [Figure 1 and 2], and with a spatula for large wounds or burn wounds.
Conclusion
The widespread and inappropriate use of antimicrobials to treat both acute and chronic wounds, coupled with spreading and systemic infections, has significantly contributed to the rise of AMR. The growing concern about AMR and its associated risks, including prolonged hospital stays and increased mortality, highlights the need for innovative antimicrobial wound care alternatives with minimal cytotoxicity, such as Flaminal®.
Flaminal® is an enzyme alginogel, designed as a singular product with multiple modes of action to comprehensively address all aspects of T.I.M.E. It effectively mitigates the complexity associated with multiple product choices and results in a more consistent and standardised approach to wound care.