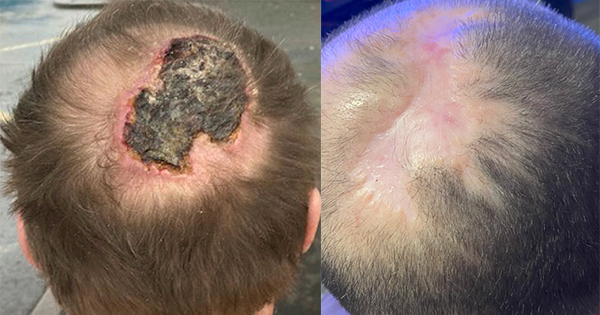
Infection is a significant problem for people with wounds and can result in delayed healing. For patients with an infected wound or those at high risk of a wound infection, knowledge of the available treatment options is vital when selecting an appropriate dressing. This article focuses on the use of Suprasorb X+PHMB in the management of critically colonised or infected wounds.
What is Suprasorb X+PHMB?
Suprasorb X+PHMB is a topical antimicrobial wound dressingfor use on lightly to moderately exuding infected or critically colonised wounds (Kingsley et al, 2009). It may also be used where wounds have been identified through a systematic assessmentto be at greater risk of infection (Dissemond et al, 2011). The wound dressing combines the antimicrobial properties of polyhexamethylene biguanide (polyhexanide, PHMB), with the ability to maintain a moist wound healing environment.
How does Suprasorb X+PHMB work?
Moisture management and bacterial control are two ofthe fundamental issues in wound management (Fumarolaet al, 2010). Suprasorb X+PHMB is composed of 4%cellulose and 96% water, which allows it to absorb exudate or donate moisture to manage exudate levels. The dressing also provides a carrier for PHMB (0.3%), which exerts itsantimicrobial effects both within the dressing, but also at thewound-dressing interface (Gray et al, 2010).
What is PHMB?
PHMB is a broad spectrum antimicrobial agent that has beenused commercially for over 60 years in a number of products, including contact lens solutions and swimming pool cleaners(Moore and Gray, 2007). It has been used in wound dressingsas an alternative to silver or honey.PHMB works by:Inhibiting bacterial cell metabolismBinding to the bacteria’s phospholipid (outer) membrane.The positively-charged PHMB molecules attach to the outer membrane of the negatively-charged bacterial cell, causing areas of dysfunction and allowing PHMB to penetrate the inner membrane. The cell increasingly is unable to control normal transmembrane ion exchange, leading to increased fluidity, permeability, loss of integrity and cell death (Gilbert,2006; Hubner and Kramer, 2010).
Safety and efficacy of PHMB
PHMB is considered to be practically non-toxic and is welltolerated with no systemic uptake detected and no reported chronic health effects. Tests of biocompatibility for PHMB(which measures its antimicrobial activity in relation to itscytoxicity) have shown that PHMB results in less damage to healthy cells than other antimicrobial agents such aschlorhexidine and povidone iodine (Hubner and Kramer, 2010).
PHMB has demonstrated good antimicrobial efficacy andhas been found to block Pseudomonas aeruginosa-induced infection (Cazzaniga et al, 2000) and prevent its degradation of wound fluid and skin proteins in vitro (Werthen et al, 2004). It can also kill a diverse range of bacteria and fungi (Lee et al, 2004).
PHMB-containing wound dressings have been shown to beactive against biofilms (Seipp and Korber, 2008; Lenselink and Andriessen, 2011) and multi-resistant pathogens includingmethicillin-resistant Staphylococcus aureus (MRSA) (Eberleinand Wild, 2008; Wild et al 2008) and vancomycin-resistantenterococci (VRE) (Shah et al, 2009).
What is HydroBalance technology?
Suprasorb X+PHMB is made up of a unique structure composedof biosynthetic HydroBalance fibres. These fibres are theproducts of a cellulose fermentation process using Acetobacterxylinium, which produce fine cellulose fibrils, providing an exceptionally large surface area. This allows the dressing to regulate the absorption and donation of moisture at the dressing interface (Alvarez et al, 2004). Surplus exudate is taken up from the wound into the dressing, while moisture is released from the dressing into the wound. Different levels of absorption and donation can be exerted within the same wound, removingexudate from one area while releasing moisture to drier areas. PHMB is not bound to the fibres of the dressing and is free to bereleased into the wound and periwound tissues. The presence offluid in the dressing means that antimicrobial activity is possible even on dry wounds (Gray et al, 2010).
Role in pain management
Pain is a common experience for patients with infected wounds(Mudge and Orsted, 2010). The moist wound environment afforded by the HydroBalance technology creates a cooling effect at the wound surface, which has been shown to reduce pain significantly (Alvarez et al, 2004). Mosti et al (2008) and Galitz et al (2009) also found that the use of Suprasorb X+PHMB saw a decrease in patient-reported pain at dressing changes, with significant reductions (p<0.05) in visual analogue scores after the first day. PHMB has also been found in laboratory studies to inhibit formation of reactive oxygen species (Gilliver, 2009). This suggests additional anti-inflammatory properties, which may play a role in pain management.
When is Suprasorb X+PHMB indicated?
Suprasorb X+PHMB is indicated for use on lightly or moderatelyexuding, superficial and deep, critically colonised and infected wounds (Kingsley et al, 2009). These may include chronic ulcers such as diabetic foot, venous leg and pressure ulcers, partial thickness wounds, surgical wounds and skin donor/recipient sitewounds. It has also been used as a safe antimicrobial alternativein the management of children with epidermolysis bullosa(Denyer, 2009) and in paediatric lacerations (Elzinga et al, 2011).Topical antimicrobials play a significant role in the prevention and management of infection. It is important that clinicians recognise and differentiate the signs and symptoms of localised, spreading and systemic infection in different wound types (EWMA, 2005).Early recognition of wounds at risk (W.A.R.) of infection is essential to avoid delayed healing and to prevent serious infections from occurring (Dissemond et al, 2010). Host susceptibility, highnumbers of bacteria in the wound and presence of devitalised or sloughy tissue can increase the risk of infection (EWMA, 2004).A checklist in the form of a score for at risk wounds has been created based on a clinically-orientated risk assessment using specific patient circumstances. A W.A.R. score of 3 or more points indicates that the wound has a greater risk of infection and local antimicrobial treatment with a topical antimicrobial such as PHMB is justified (Dissemond et al, 2011).
How to apply Suprasorb X+PHMB?
- Prepare the woundThoroughly cleanse the wound according to local protocols.Ensure the surrounding skin is clean and dry.
- Apply the dressingSelect an appropriately sized dressing. Remove the protectivefilm from both sides and apply either side down. If required,the dressing and the rope may be cut to size with sterilescissors, or folded to conform to the shape of the wound.The dressing should overlap the wound by 2-3cm. Apply a suitable secondary dressing as indicated by the level ofexudate. Film dressings are recommended as a first choice,particularly when there are low levels of exudate.
Tip: When using the rope, gently place it into the wound,layering by folding it onto itself. In cavities, ensurethat 2-3cm of the rope dressing is secured outside ofthe wound for easy removal. Do not pack the materialtoo tightly within the wound. Cover the rope with anappropriate secondary dressing
Tip: When using the sheet dressing, gently smooth thedressing in place and allow it to conform to theshape of the wound
How frequently should the dressings be changed?
All wounds should be checked at regular intervals to monitor for improvement or deterioration. More frequentreview is advised for infected wounds and daily dressing changes performed as appropriate. For wounds that are at risk of invasive infection (i.e. critically colonised), the frequency of changing Suprasorb X+PHMB sheet or rope dressing is dependent on the status of the wound bed and can range between 2-7 days.
Tip: When removing Suprasorb X+PHMB sheet dressing lift one edge and gently peel the dressing from the wound.Should the dressing dry out, rehydrate the dressing withsaline 30 minutes before removal.
When should Suprasorb X+PHMB be discontinued?
As with all topical antimicrobial treatments, therapy should be reviewed after two weeks with the decision to continue treatment based on individual patient assessment. When there is a deep infection suspected, systemic antibiotic therapy must be considered (Best Practice Statement, 2011).
When is Suprasorb X+PHMB contraindicated?
Suprasorb X+PHMB is not indicated for full thickness burns, cartilage injuries or for intraocular applications. It should not be used in patients with a known sensitivity to PHMB.
What is the evidence for use?
The clinical effectiveness of Suprasorb X+PHMB has been reported through a number of clinical trials (Table 1) and numerous case studies (Glover and Wicks, 2009 ; Gray et al, 2011).
Availability on Drug Tariff
Suprasorb X+PHMB is available on prescription in a range of sizes (5cmx5cm; 9cmx9cm; 14cmx20cm), including a rope dressing (2cmx21cm) for cavity wounds.
Summary
Suprasorb X+PHMB combines a broad spectrum of antimicromicrobial activity and low cytotoxicity with an ability to absorb and release moisture to maintain an optimum moist wound healing environment. This has been shown to reduce wound pain during treatment and provides an alternative option to silver or honey when treating patients with an infected wound or patients who are at risk of infection.
AUTHOR DETAILS
Andrew Kingsley[1], Martin Kiernan[2]
- Clinical Manager, Infection Control and Tissue Viability, North Devon District Healthcare NHS Trust
- Nurse Consultant, Prevention and Control of Infection, Southport and Ormskirk Hospital NHS Trust
References
- Alvarez OM, Patel MM, Booker J, Markowitz L (2004) Effectiveness of a biocellulose wound dressing for the treatment of chronic venous leg ulcers: results of a single-centre randomized study involving 24 patients. Wounds 16(7):224-33
- Best Practice Statement (2011) The use of topical antiseptic/antimicrobial agents in wound management. 2nd edition. Wounds UK, London.
- Cazzaniga A, Serralta V, Davis S et al (2000) The effect of antimicrobial gauze dressing impregnated with 0.2% polyhexamethylene biguanide (PHMB) as a barrier to prevent Pseudomonas aeruginosa wound invasion. Wounds 14:169-76
- Denyer J (2009) Management of the infant with epidermolysis bullosa. Infant 5(6):168-71
- Dissemond J, Gerber V, Kramer A, et al (2010) A practice-orientated recommendation for treatment of critically colonized and locally infected wounds using polihexanide. J Tissue Viability 19(3):106-15
- Dissemond J, Assadian O, Gerber V, et al (2011) Classification of wounds at risk and their antimicrobial treatment with polihexanide: A practice-oriented expert recommendation. Skin Pharmacology Physiology 24:245-55
- Eberlein T, Wild T (2008) Approaches to therapy and an outlook on improving the clinical data situation, with a special focus on the polihexanide-containing Suprasorb X+PHMB Hydrobalanced dressing. EWMA conference extended abstract. Available at: http://www.lohmann-rauscher.com/files/1c35745a596ebd89bcd8e12205aca04a/1018/Summary_ICW_-_DGfW_-_EWMA_English.pdf#page=27 [accessed Oct 2011]
- Elzinga G, van Doorn J, Wiersema AM, et al (2011) Clinical evaluation of a PHMB-impregnated biocellulose dressing on paediatric lacerations. J Wound Care 20(6):280-6
- EWMA Position Document (2005) Identifying criteria for wound infection. MEP Ltd, London
- EWMA Position Document (2004) Wound bed preparation in practice. MEP Ltd, London
- Fumarola S, Butcher M, Cooper P et al (2010) A clinical audit of Suprasorb® X+PHMB. Wounds UK 6(3):78-87
- Galitz C, Hammerle G, Signer M (2009) Polihexanide versus silver wound dressings: first interim results of a controlled, randomized, prospective multicenter study. Poster. European Wound Management Association (EWMA) Helsinki/FIN, 20-22 May 2009. EWMA J Supplement 9(3):178-86
- Gilbert P (2006) Avoiding the resistance pitfall in infection control – does the use of antiseptic products contribute to the spread of antibiotic resistance? Ostomy Wound Manage 52: 10A(suppl);1S-3S
- Gilliver S (2009) PHMB: a well-tolerated antiseptic with no reported toxic effect. J Wound Care. Activa Healthcare Supplement
- Glover D, Wicks G (2009) Suprasorb X+PHMB: the clinical evidence. J Wound Care. Activa Supplement
- Gray D and Cooper P. Effective management of wound infection and quality of life with Suprasorb® X+PHMB. Wounds UK, London 2011.
- Gray D, Barrett S, Battacharyya M et al (2010) PHMB and its potential contribution to wound management. Wounds UK 6(2): 40-6
- Hubner NO, Kramer A (2010) Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol Physiol 23(suppl 1):17-27
- Kingsley A, Tadej M, Colbourn A, et al (2009) Suprasorb X+PHMB: antimicrobial and HydroBalance action in a new wound dressing. Wounds UK 5(1):72-7
- Lee WR, Tobias KM, Bernis DA, Rohrback BW (2004) In vitro efficacy of a polyhexamethylene biguanide-impregnated gauze dressing against bacteria found in veterinary patients. Vet Surg 33(4):404-11
- Lenselink E, Andriessen A (2011) Clinical effectiveness of polihexanide on biofilms in wounds. Poster presentation at AIUC, Ancona, Italy
- Moore K, Gray D (2007) Using PHMB antimicrobial to prevent wound infection. Wounds UK 3(2):96-102
- Mosti G, Mattaliano V, Schmitz M, Abel M (2008) Successful therapy of critically colonized or locally infected wounds with a new HydroBalance biocellulose-based wound dressing with polihexanide on outpatients. Poster presentation. Wounds UK, Harrogate
- Mudge E, Orsted H (2010). Wound infection and pain management made easy. Wounds International 1(3). Available at: www.woundsinternational.com [accessed Oct 2011]
- Shah C, Hanson P, Swaniker BS et al (2009) Efficacy and mode of action of a new PHMB impregnated polyurethane foam dressing. Data on file. Available at http://www.forumenfermagem.org/newsletter/images/H6409_Kendall_AMD_Foam_MOA_WP_F.pdf [accessed Oct 2011]
- Seipp HM, Korber A (2008) Biofilm, fibrin, resistance: antibacterial measures with a focus upon polihexanide. In: Polihexanice – an antimicrobial substance with various properties – for critical colonised or local infected wounds. Lohmann & Rauscher, Neuwield, Germany
- Werthern M, Davoudi M, Sonesson A e al (2004) Pseudomonas aeruginosa-induced infection and degradation of human wound fluid and skin proteins ex vivo are eradicated by a synthetic cationic polymer. J Antimicrob Chemother 54(4):772-9
- Wild T, Buckner M, Payrich M, et al (2009) Prospective, randomized study for eradication of MRSA with polihexanide containing biocellulose dressing compared with polihexanide wound solution. Poster presentation, EWMA, Helsinki